Biological Molecules
Water and transport
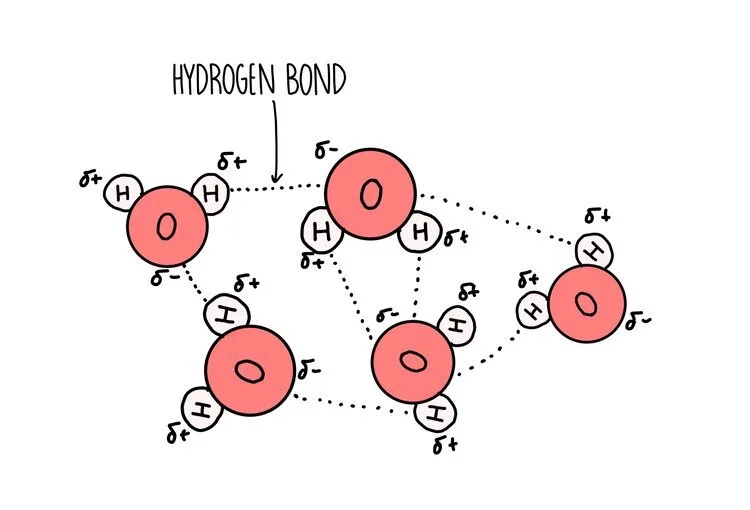
Water is a polar molecule - this means that the electrons are shared unequally within the bonds that hold a water molecule together. Oxygen is greedy for electrons so pulls the electrons that make up the single covalent bond closer towards it (it is electronegative). This gives oxygen a slight negative charge and hydrogen a slight positive charge.
A hydrogen bond forms between the slightly negative oxygen atom of one water molecule and the slightly positive hydrogen atom on another molecule. The ability of water molecules to hydrogen bond gives it some special properties:
It is an excellent solvent: water is a good solvent because it can form hydrogen bonds with other polar molecules or charged ionic compounds. Water dissolves more substances than any other liquid so it is referred to as the ‘universal solvent’. This is handy for humans, since our blood consists mostly of water which is able to dissolve hydrophilic molecules such as glucose, ions and some amino acids.
It has a high latent heat of evaporation - this means that a lot of energy is used to convert water from a liquid to a gas. This is why we feel cooler when we sweat, since the evaporation of water from our skin’s surface takes a lot of heat energy with it.
It has a high specific heat capacity - all those hydrogen bonds within water are great at absorbing energy, which means you have to add a lot of it to heat water up. This is really useful for marine life since it means that bodies of water are fairly resistant to changes in temperature, making it a stable habitat in which to live.
It is cohesive – water molecules have a tendency to stick to other water molecules (cohesion) because the slightly positive hydrogen of one water molecule attracts the slightly negative oxygen on another water molecule. Cohesion helps water to flow, making it a good transport medium.
It can insulate large bodies of water – solid water (ice) is less dense than liquid water because the water molecules in ice are held further apart. This is because each water molecule forms four hydrogen bonds, connecting it in a lattice shape. This means that in the colder months, ice forms an insulating later so that the water beneath doesn’t freeze, allowing aquatic organisms to survive.
Carbohydrates
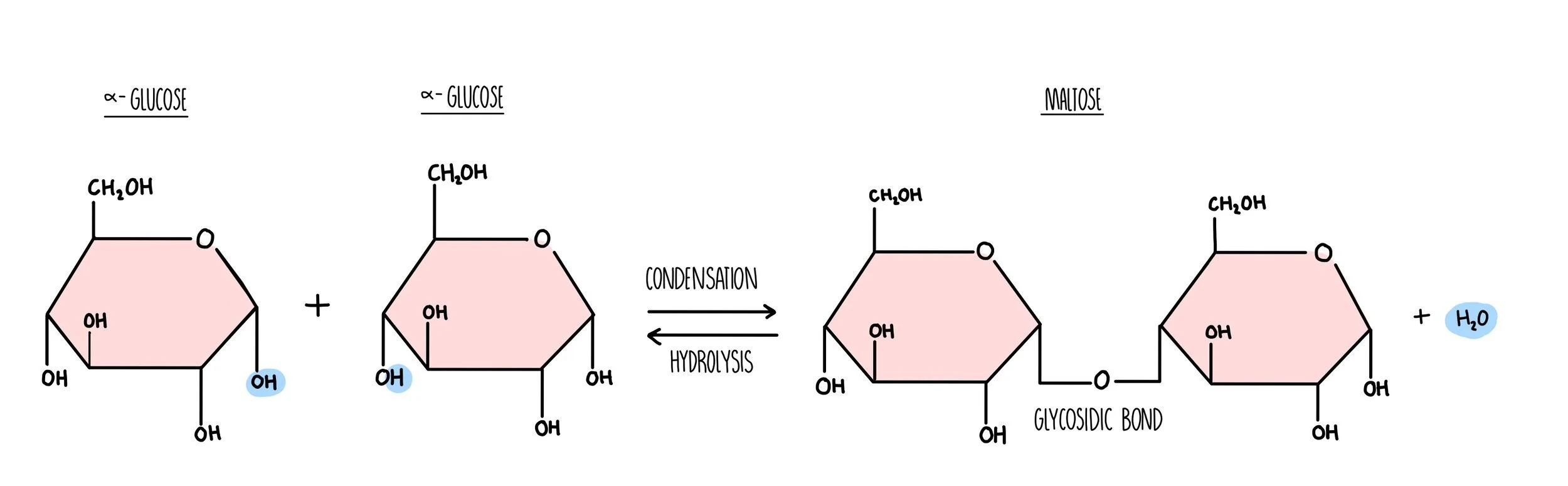
Carbohydrates are long chains of sugar molecules (the technical term is ‘polysaccharides’) formed from lots of individual sugar molecules (monosaccharides) joining together. When two monosaccharides react to form a disaccharide, a condensation reaction occurs. During the condensation reaction, a molecule of water is removed (from a hydroxyl group on one sugar and a hydrogen on another) and a glycosidic bond forms. When polysaccharides are broken down during digestion, a hydrolysis reaction occurs in which a water molecule is added to break the glycosidic bond.
Carbohydrates are made up of carbon, hydrogen and oxygen only. Monosaccharides such as glucose and fructose consist of a ring structure with the general formula CH2O. Glucose is a hexose sugar, which means it is made up of six carbon atoms. It can exist as two different forms, alpha and beta which differ in the position of the hydrogen and hydroxyl groups on the right-hand carbon. An easy way to tell the difference between them is:
Alpha - the hydrogen atom is above the carbon.
Beta - the hydrogen atom is below the carbon.
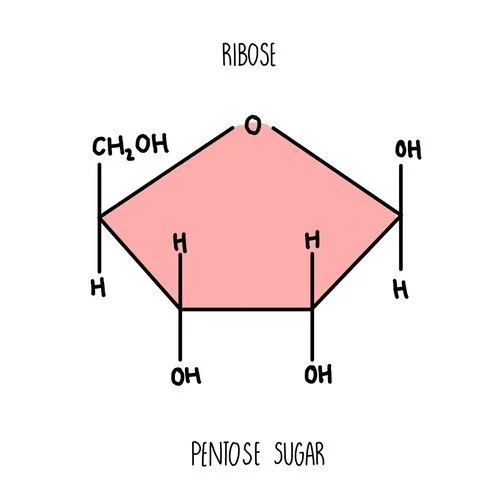
Ribose is another example of a monosaccharide - it’s a component of RNA molecules. It is a pentose sugar with five carbon atoms in the ring.
Polysaccharides
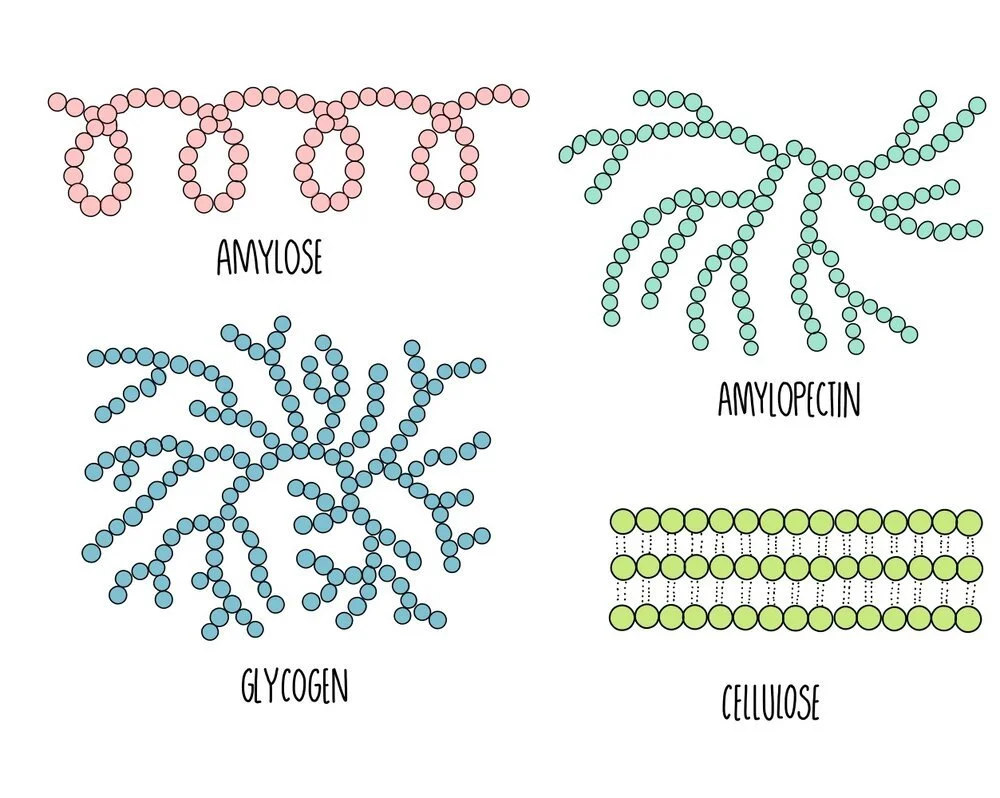
The polysaccharides found in plants are starch and cellulose. Starch is broken down by the plant when it needs energy and cellulose is the major component of plant cell walls. Starch exists in two different forms: amylose and amylopectin. In animals, the energy storage carbohydrate is glycogen.
Amylose: unbranched spiralling chains of alpha-glucose molecules. Its coiled structure means that it is very compact so lots of amylose can be packed into a cell.
Amylopectin: branched chains of alpha-glucose molecules. Its branches increase its surface area which means that enzyme can quickly break it apart when glucose is needed for respiration.
Cellulose: long unbranched chains of beta-glucose molecules. Multiple chains are linked together by hydrogen bonding to form strong structures called micofibrils. The strong microfibrils in the cell wall help to give plant cells their shape and structural support.
Glycogen: branched chains of alpha glucose, similar to amylopectin but with more side-branches. This gives it a large surface area for enzyme action to release glucose when energy is needed. It is more compact that amylopectin, which means more can be stored in a cell.
Lipids
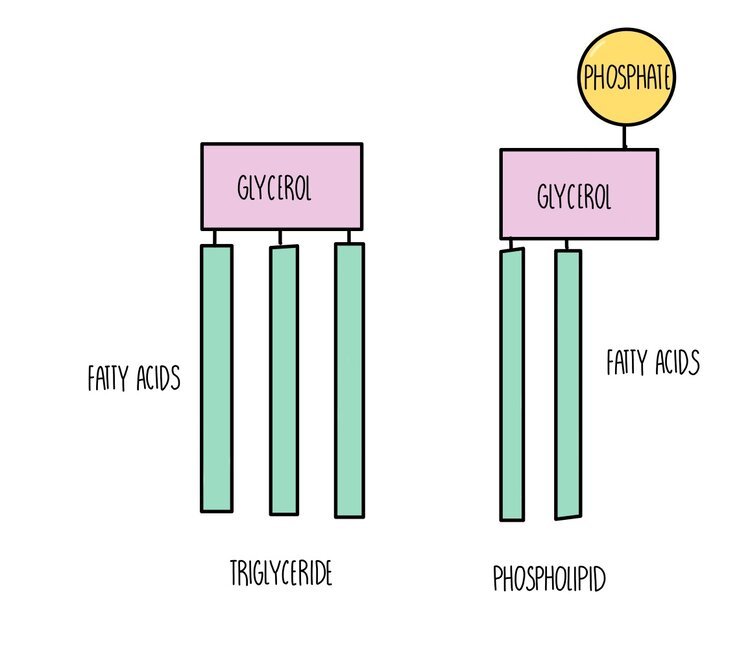
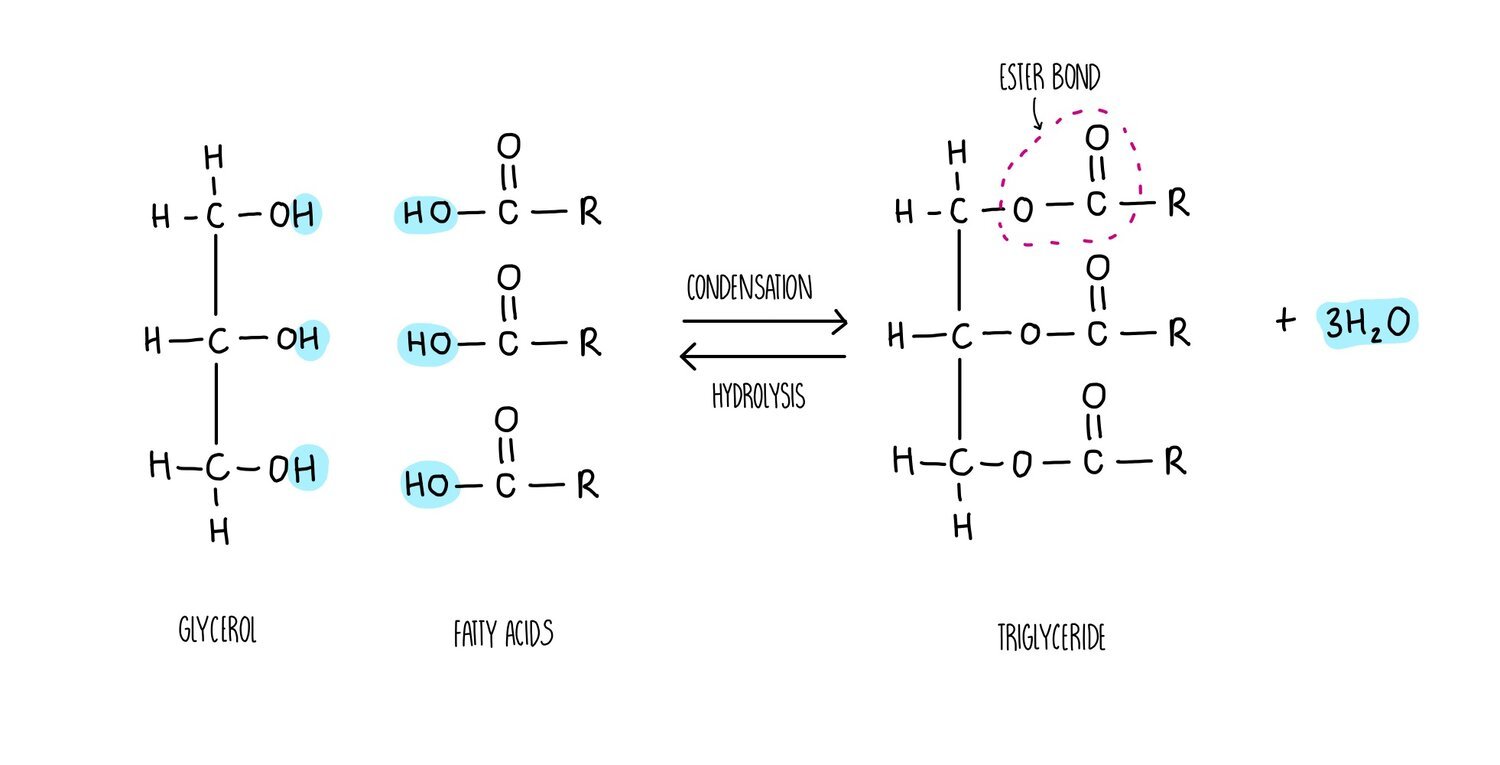
Triglycerides, phospholipids and cholesterol are all types of lipid. Triglycerides and phospholipids have similar structures as they are both made up of glycerol connected to fatty acids molecules through ester bonds. However, triglycerides contains three fatty acid ‘tails’ whereas phospholipids only have two. Phospholipids also possess a phosphate group which is absent in triglycerides. The fatty acid tails of triglycerides or phospholipids can be saturated (only single carbon-carbon bonds) or unsaturated (contain at least one double carbon bond).
The synthesis of a phospholipid or triglyceride from glycerol and fatty acids involves the formation of an ester bond. Just like we saw for the formation of a glycosidic bond, the synthesis of ester bonds is a condensation reaction in which a water molecule is released. Breaking apart a triglyceride or phospholipid involves the addition of water in a hydrolysis reaction.
Different types of lipids have different functions in living organisms:
Triglycerides: used as an energy store. A lot of energy is released when the ester bonds are hydrolysed - around twice as much compared to the breakdown of carbohydrates. Inside cells, triglycerides group together into lipid droplets where the hydrophobic tails face inwards and the hydrophilic heads face outwards. These insoluble droplets make good energy storage molecules since they do not affect the osmotic potential of the cell.
Phospholipids are the main component of cell membranes. They form a phospholipid bilayer with the hydrophobic tails facing each other and the hydrophilic heads facing outwards, forming a barrier to prevent any polar molecules from entering or leaving the cell.
Cholesterol is also found in cell membranes and helps to strengthen the membrane. Cholesterol pushes the hydrophobic tails of phospholipids closer together, making the membrane more rigid.
Proteins
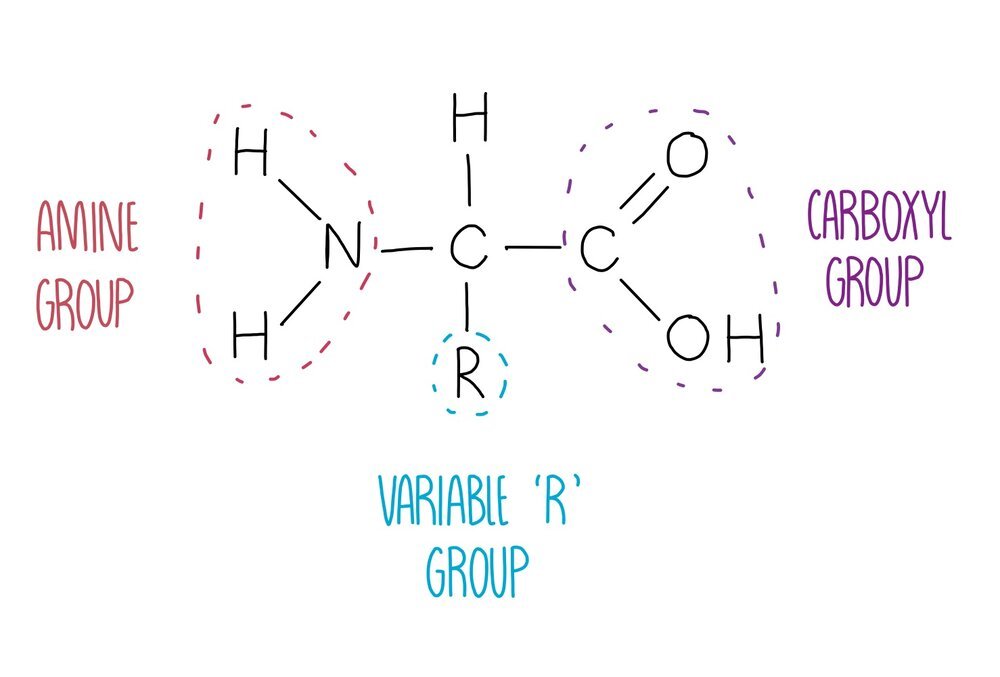
Proteins are polymers of amino acids joined together by peptide bonds. There are 20 different amino acids that our body needs to stay healthy, all of which have the same general structure. They consist of a central carbon atom attached to four different groups: an amine group, a hydrogen atom, a carboxyl group and an 'R’ group which is different in each amino acid. The identity of the R group will influence how the amino acid interacts with other amino acids, therefore influencing protein folding. For example, lysine has a charged R group and is able to form ionic bonds with negatively charged amino acids.
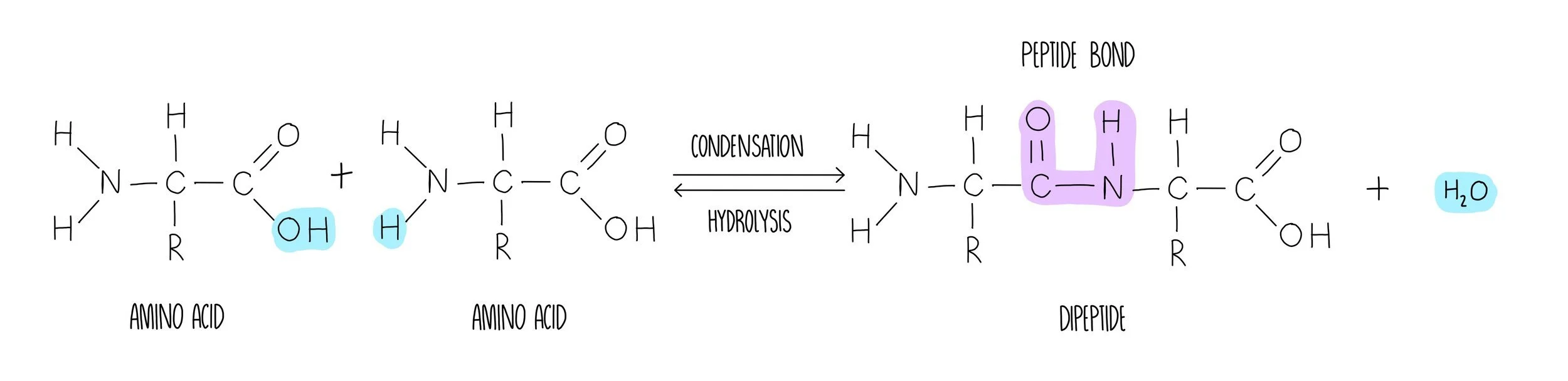
Peptide bond formation involves the removal of water in a condensation reaction. The water is formed by removing a hydrogen atom from the amine group and a hydroxyl (-OH) group from the carboxylic acid. What’s left behind is a peptide bond (-CONH) which can be broken with the addition of water in a hydrolysis reaction.
Once a long polypeptide chain is synthesised from the formation of numerous peptide bonds between adjacent amino acids, the polypeptide undergoes multiple stages of folding. These stages of protein origami are defined as the primary, secondary, tertiary and quaternary structures which describe a protein from its most unravelled to its final compact structure.
Primary structure: peptide bonds have formed between amino acids to form a long, straight chain (polypeptide).
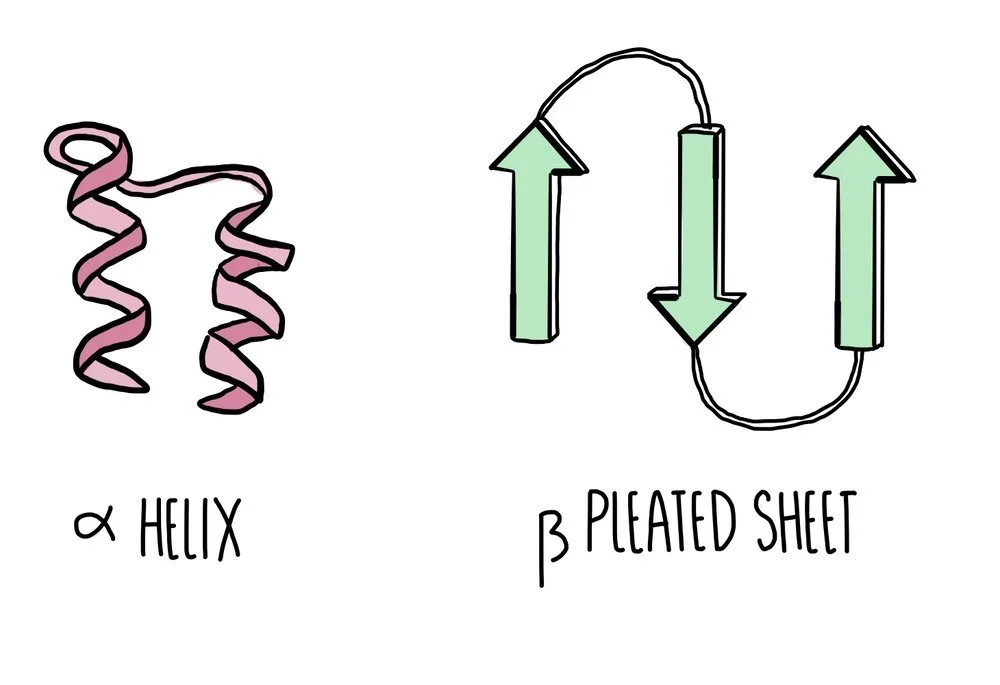
Secondary structure: hydrogen bonds form between nearby amino acids (from the amine group on one amino acid to the carboxyl group of another) to form either an alpha helix or a beta pleated sheet. Proteins which form neither of these two structures will form a random coil.
Tertiary structure: more bonds form between the different R groups to give the protein a 3D structure. R-group interactions involve hydrogen bonds, disulfide bonds, ionic bonds and polar interactions. If proteins are made of a single polypeptide chain, this is their final overall structure.
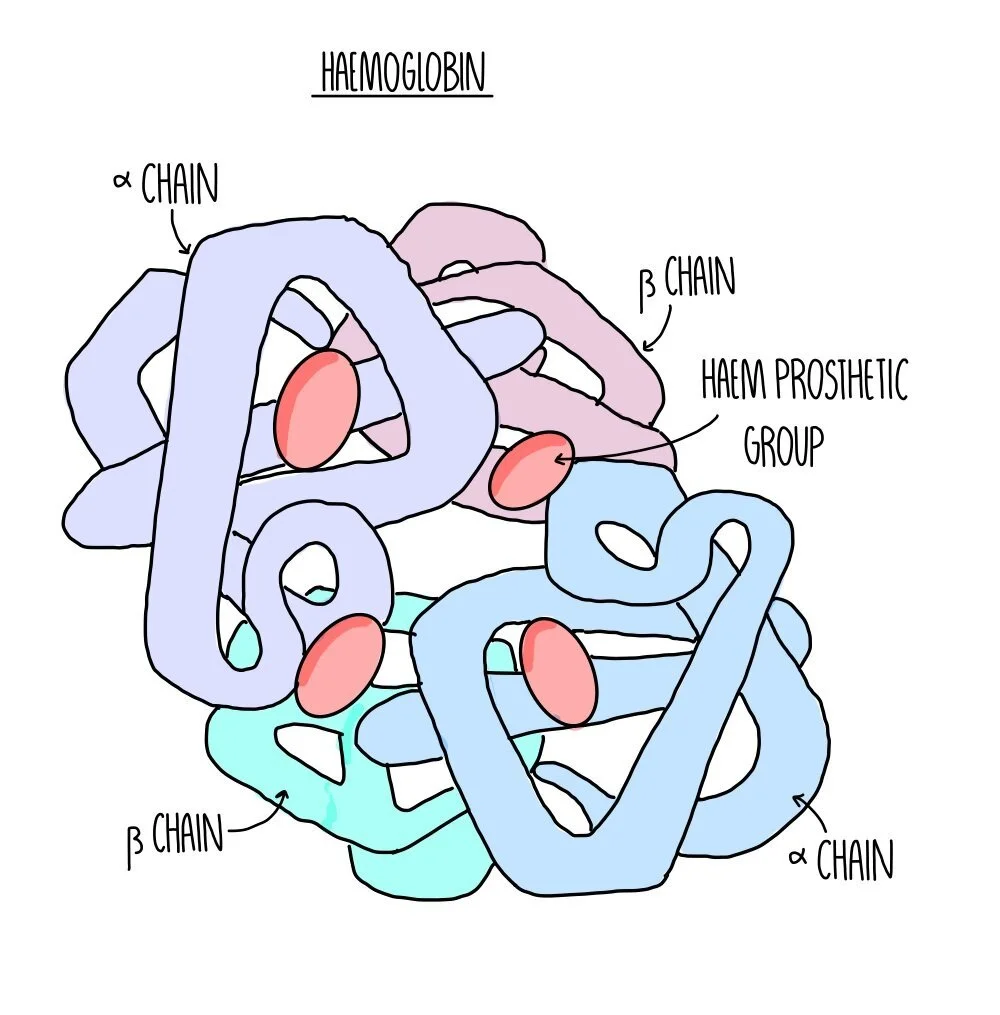
Quaternary structure: this is the structure formed from the interaction of multiple polypeptide chains held together by bonds. Haemoglobin is an example of a protein with quaternary structure. It consists of four polypeptide chains (two alpha chains and two beta chains) bonded together. Each chain surrounds an iron-containing haem group. The haem group is referred to as a prosthetic group - non-protein components which are required for protein function.
Globular vs fibrous proteins
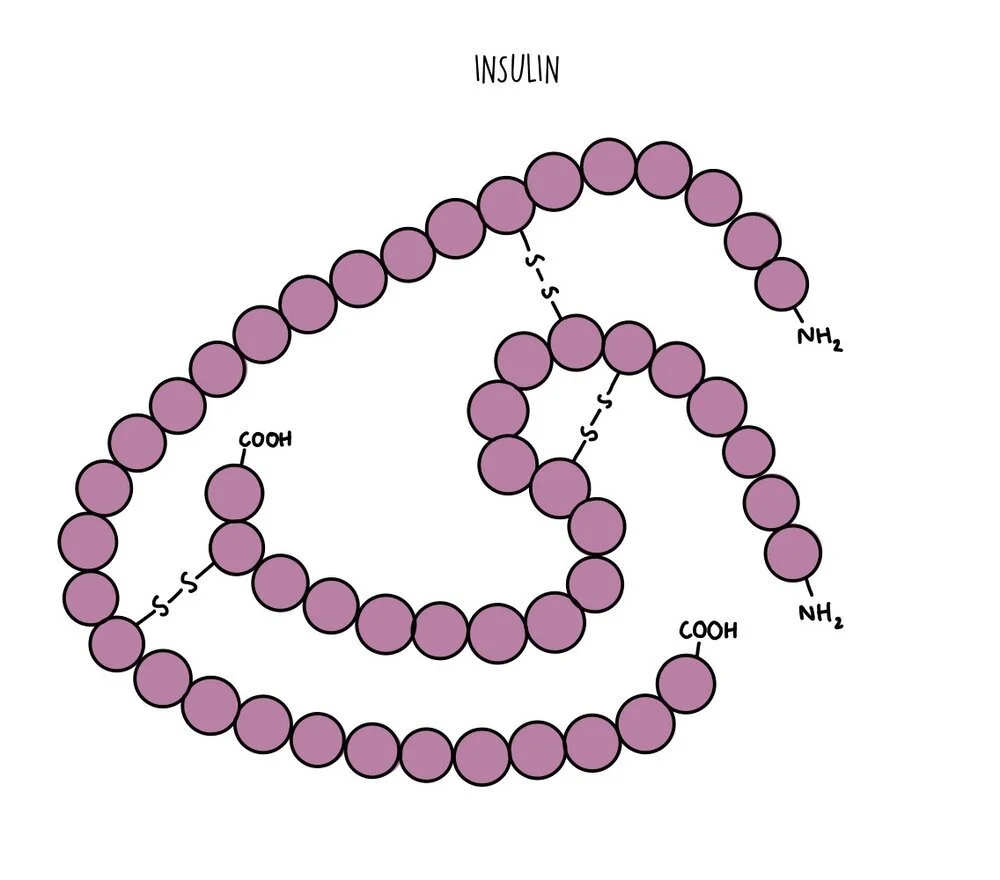
All proteins can be organised into one of two broad categories: globular proteins and fibrous proteins. Globular proteins are spherical and arranged with their hydrophobic amino acids tucked inside and the hydrophilic amino acids exposed on the outside. This means globular proteins are soluble and can be transported easily from one part of the cell to another. They perform functional roles and include things like enzymes (such as amylase), hormones (such as insulin) or proteins like haemoglobin. Globular proteins unravel and denature when the temperature or pH deviates from optimum levels. Globular proteins with prosthetic groups attached (such as haemoglobin) are referred to as conjugated proteins.
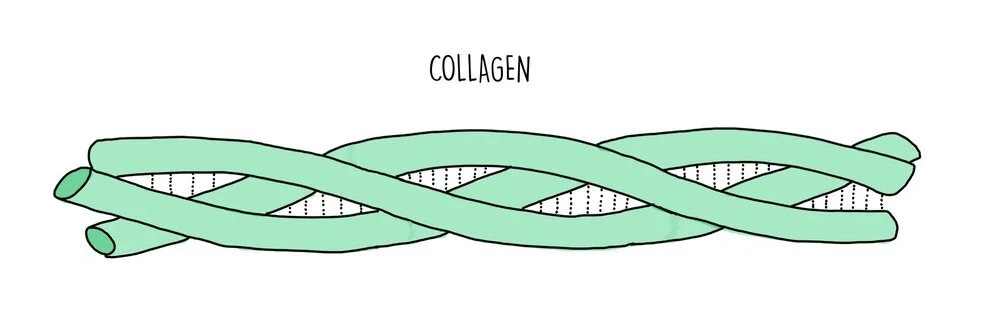
Fibrous proteins are long and thin and their primary structure consists of a repetitive sequence of amino acids. They perform structural roles so they are strong and insoluble. Examples of fibrous proteins include collagen, keratin and elastin. Collagen consists of three polypeptide chains wrapped tightly around each other to form a stable quaternary structure held together by numerous hydrogen bonds. Collagen is found in connective tissue, such as skin, muscle and bone. Fibrous proteins tend to be less sensitive than globular proteins to changes in temperature and pH.
Inorganic ions
There is an array of cations (positive ions) and anions (negative ions) which are necessary for many biological processes:
- Calcium ions, Ca2+: required for the transmission of action potentials, the formation of bone, the release of insulin from the pancreas and as an enzyme cofactor.
- Sodium ions, Na+: required for the generation of action potentials, muscle contraction and for maintaining blood pressure.
- Potassium ions, K+: required for the generation of action potentials, muscle contraction, maintaining blood pressure and for activating photosynthesising enzymes in plants.
- Hydrogen ions, H+: important role in respiration and photosynthesis reactions and influences pH of a cell by increasing acidity.
- Ammonium ions, NH4+: an important part of the nitrogen cycle. It is absorbed by plants and incorporated into amino acids for protein synthesis.
- Nitrate ions, NO3-: an important part of the nitrogen cycle. It is absorbed by plants and incorporated into amino acids for protein synthesis.
- Hydrogencarbonate ions, HCO3-: maintains constant pH in the blood by acting as a buffer.
- Chloride ions, Cl-: maintains constant pH in the blood through the process of 'chloride shift' and acts as a cofactor for the enzyme amylase.
- Phosphate, PO43-: used to synthesise biological molecules such as nucleic acids and phospholipids and is added to enzymes and other proteins to regulate their activity
- Hydroxide, OH-: changes the pH of a cell by making the area more alkaline.
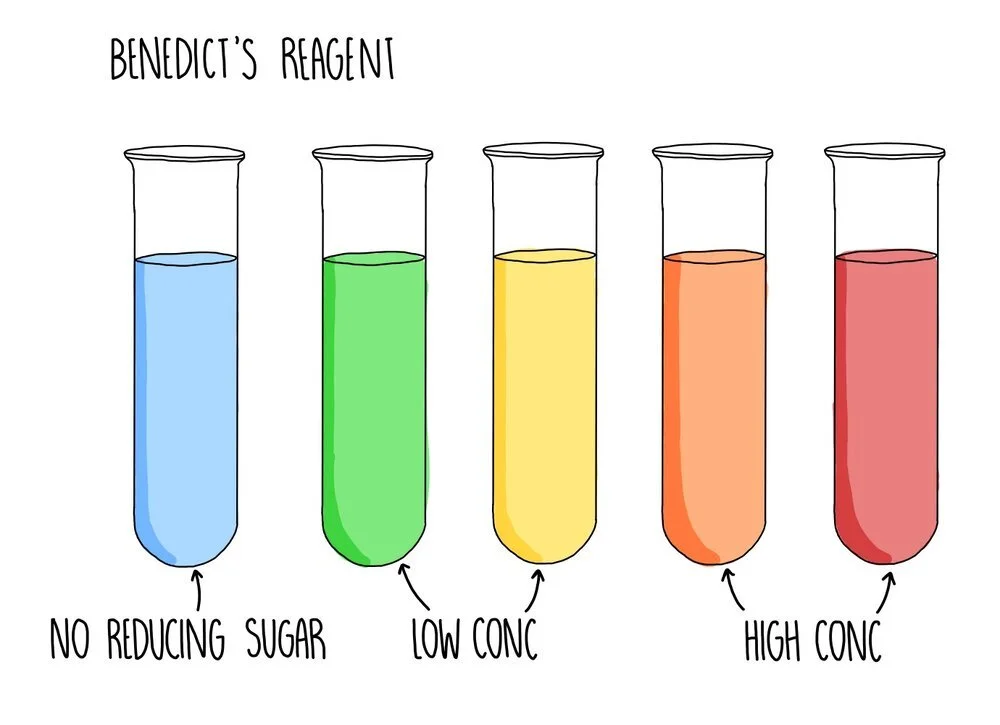
Testing reducing sugars using Benedict’s solution
A reducing sugar is any sugar that can act as a reducing agent because of its aldehyde or ketone group. All monosaccharides are reducing sugars, along with some disaccharides (such as maltose and lactose). If the sugar you are testing is a reducing sugar, it will change colour from blue to green/yellow/orange/brick red, depending on the concentration of sugar you are testing. Low concentrations of reducing sugar will cause a green or yellow precipitate to form whereas a reducing sugar of higher concentration will appear orange or brick red.
If the test for reducing sugars is negative and you want to confirm the presence of a non-reducing sugar, such as sucrose, you need to break down the sample into monosaccharides using hydrochloric acid which breaks the glycosidic bonds. The sample is neutralised with sodium hydrogencarbonate and the Benedict’s test can be carried out. Since sucrose will have been broken down into glucose and fructose (both reducing sugars), you should now see a positive result if your original sample contained sucrose.
If you want to accurately measure the amount of reducing sugar in a sample, you can use calorimetry. Calorimetry works by measuring how much light is able to pass through the solution. The less light that passes through, the higher the absorbance of the sample and the higher the concentration. Before measuring the unknown sample, you first need to measure the absorbance readings of a series of solutions of a known concentration. You would plot a calibration curve of absorbance against concentration then measure the absorbance of the sample of unknown concentration. You can read off your graph to find the concentration which corresponds to this particular absorbance reading.
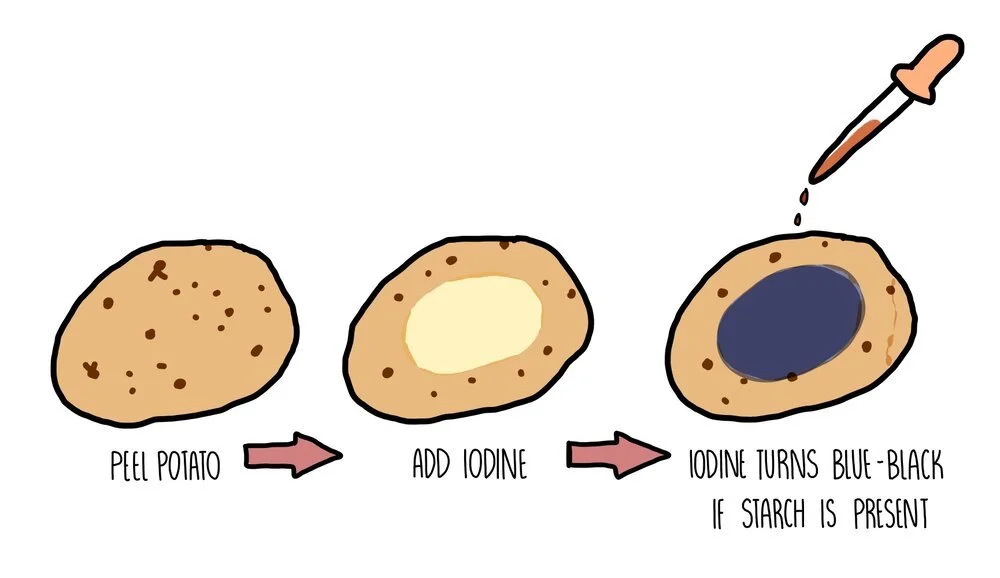
Testing for starch using iodine
Starch is the storage carbohydrate in plant cells. If you want to test a part of a plant (such as a potato) for starch, you need to add iodine dissolved in potassium iodide solution. A positive result occurs when the solution changes colour from orange/brown to blue/black.
Testing for lipids using the Emulsion test
To test for the presence of lipid in a sample, you need to add ethanol to a sample and mix thoroughly by shaking. Add an equal volume of water and if lipid is present a milky white suspension should form. If the solution remains colourless, no lipid was present in the sample.
Testing for proteins using Biuret reagent
The Biuret test involves adding sodium hydroxide to a sample followed by copper sulfate solution (together these compounds are referred to as Biuret reagent). A colour change from blue to purple indicates a positive result.
Biosensors
A biosensor is an analytical device that is used to detect the presence of a chemical. It uses biological molecules (e.g. an enzyme) to produce a chemical signal, which is converted into an electrical signal by a transducer, which is then processed.
For example, glucose biosensors are used to determine the concentration of glucose in a solution, using the enzyme glucose oxidase. Glucose oxidase catalyses the oxidation of glucose at the electrodes, creating a charge. The electrodes (the transducer) convert the charge into an electrical signal which is processed to determine glucose concentration.
Chromatography
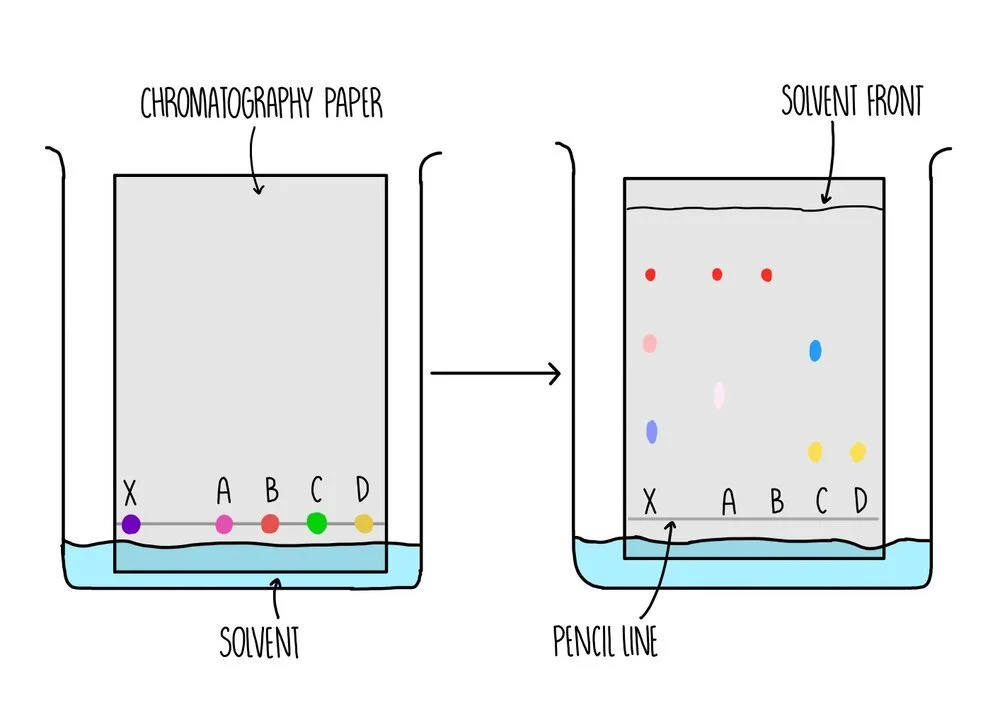
Paper chromatography is used to separate a mixture of soluble substances, such as different amino acids in a mixture. It involves a stationary phase (the part of the equipment which doesn’t move) which is a sheet of paper and a mobile phase (the thing which moves) which is the solvent.
A line is drawn near to the bottom of the piece of paper - this is the starting line where the amino acid mixture will be placed. It is important that the line is drawn in pencil because the ink from pens will also dissolve in the solvent and cause a mess.
The paper is placed in a tank containing some solvent and a lid is placed on top of the tank to prevent evaporation of the solvent. The solvent need to be below the starting line so that the mixture does not dissolve in the solvent before they can move along the paper.
As the paper absorbs the solvent, the solvent moves further up the stationary phase. The amino acids are carried up the paper along with the solvent with different dyes moving up the paper to different extents. The total distance travelled by the solvent is the solvent front.
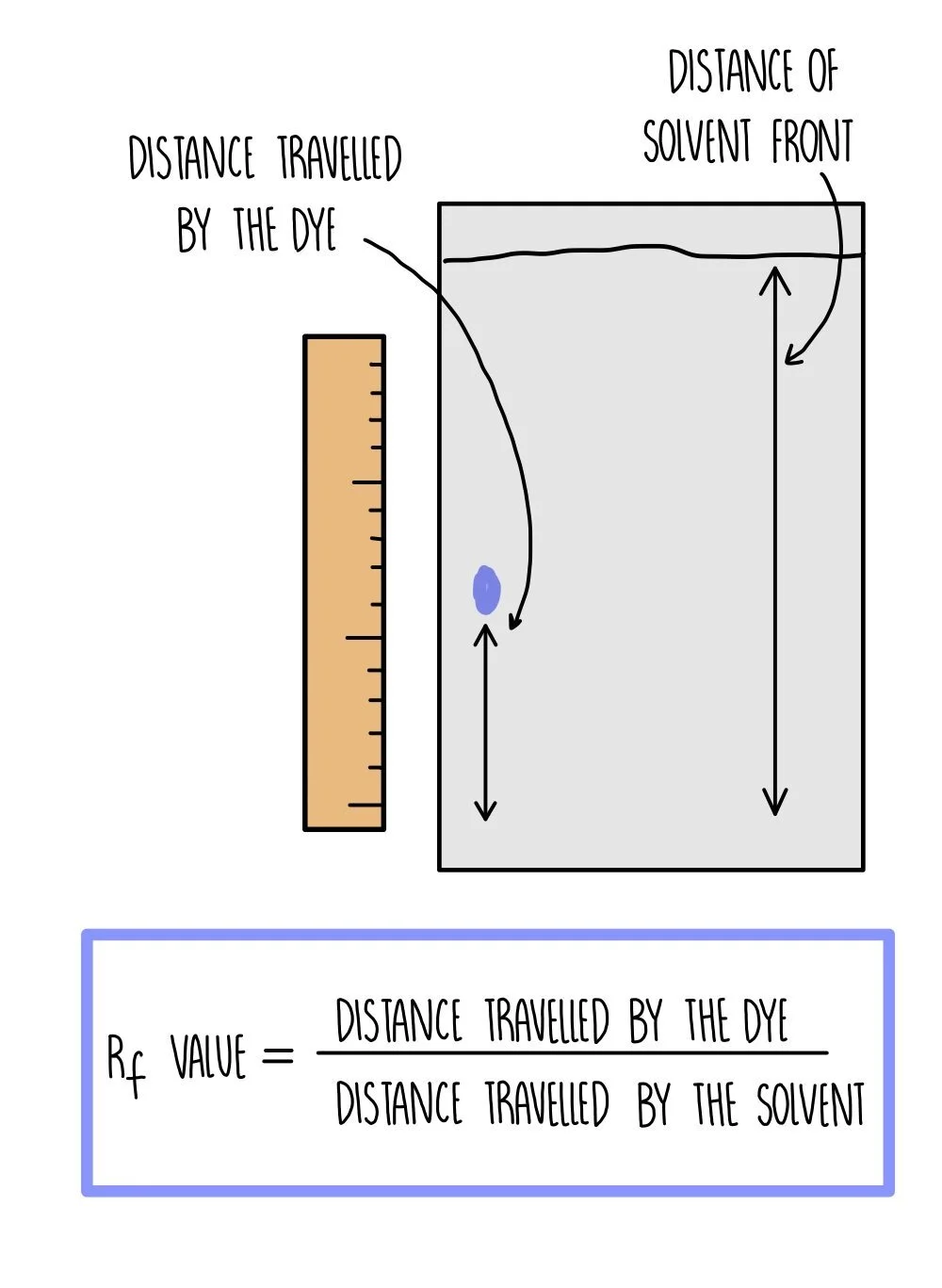
An Rf value is calculated for each amino acid using the equation:
Rf = distance travelled by dye / distance travelled by solvent
Because different compounds have different Rf values, we can use chromatography to identify the compounds present by comparing their Rf value to the published values in a data book.