Cloning and Biotechnology
Natural plant clones
Some plants, such as strawberries, can reproduce asexually in a process called vegetative propagation. It involves the non-reproductive tissues (e.g. roots, stems and leaves) of the plant. Gardeners can take advantage of vegetative propagation as a cheap and easy method of cloning plants. This could involve taking cuttings or grafting (joining the shoot of one plant to the growing stem of another plant).
Cloning plants from cuttings:
Use a scalpel to cut a shoot (5-10 cm) from the parent plant.
Remove the leaves from the lower end of the shoot but leave one at the shoot tip.
Dip the end of the shoot in rooting powder – this contains hormones which promote root growth.
Plant the cutting in compost and keep warm (either by placing in a propagator or by covering the pot with a plastic bag).
After a few weeks of growth, the cutting is ready to plant. It will be genetically identical to the parent plant.
Artificial plant clones
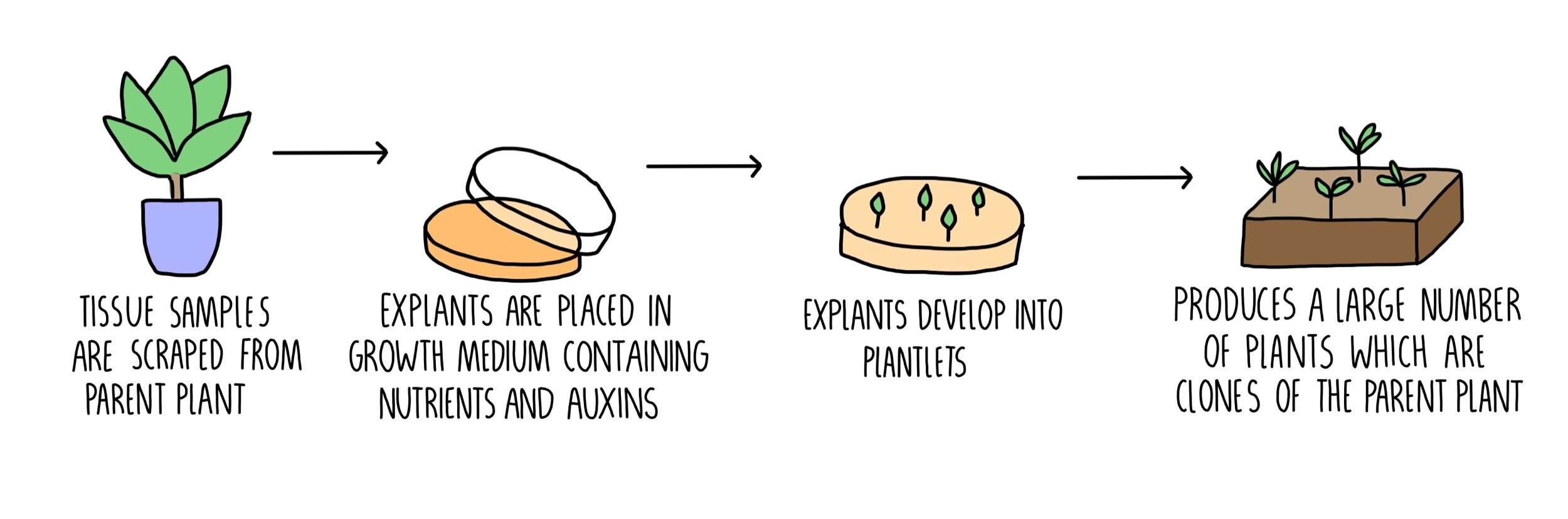
Plants can also be cloned artificially, using a method called tissue culture. To do this, you need to carry out the following steps:
Using a sterile scalpel, scrape off some cells from the parent plant. You’ll want to do this from the stem and root tips (meristems) as this is where the stem cells are found.
Cells are sterilised to kill any microorganisms which would compete with the plant and reduce its growth.
Cells are transferred to a culture medium containing growth hormones (e.g. auxin) and nutrients (e.g. glucose).
Once the cells have grown into a small plant, they are planted in compost. They will all be clones (genetically identical) to the parent plant.
Tissue culture is a good way of producing lots of plants quickly and cheaply which can be carried out all year round. It is especially useful for plants that don’t readily reproduce or are endangered.
Pros of plant cloning:
Can be carried out independently of the seasons – sexual reproduction can only take plant when the plant is fertile (usually the spring and summer months)
Any desirable characteristics in the parent plant will be replicated in the cloned plants.
Requires less space than conventional growing methods.
Quick and inexpensive.
Cons of plant cloning:
Clones are genetically identical so equally vulnerable to changes in the environment e.g., disease or drought.
Plants growing in tissue culture could die if they are infected by pathogenic microorganisms.
Any undesirable characteristics in the parent plant will be replicated in the cloned offspring.
High production costs due to the energy required and training.
Animal cloning
Cloning in animals can also occur naturally or artificially. An example of natural clones is the formation of monozygotic (identical) twins, which occur when a single fertilised egg, or zygote, divides then splits and develops into two separate embryos. Animal clones can be artificially produces by one of two methods: Artificial embryo twinning and somatic cell nuclear transfer.
Artificial embryo twinning
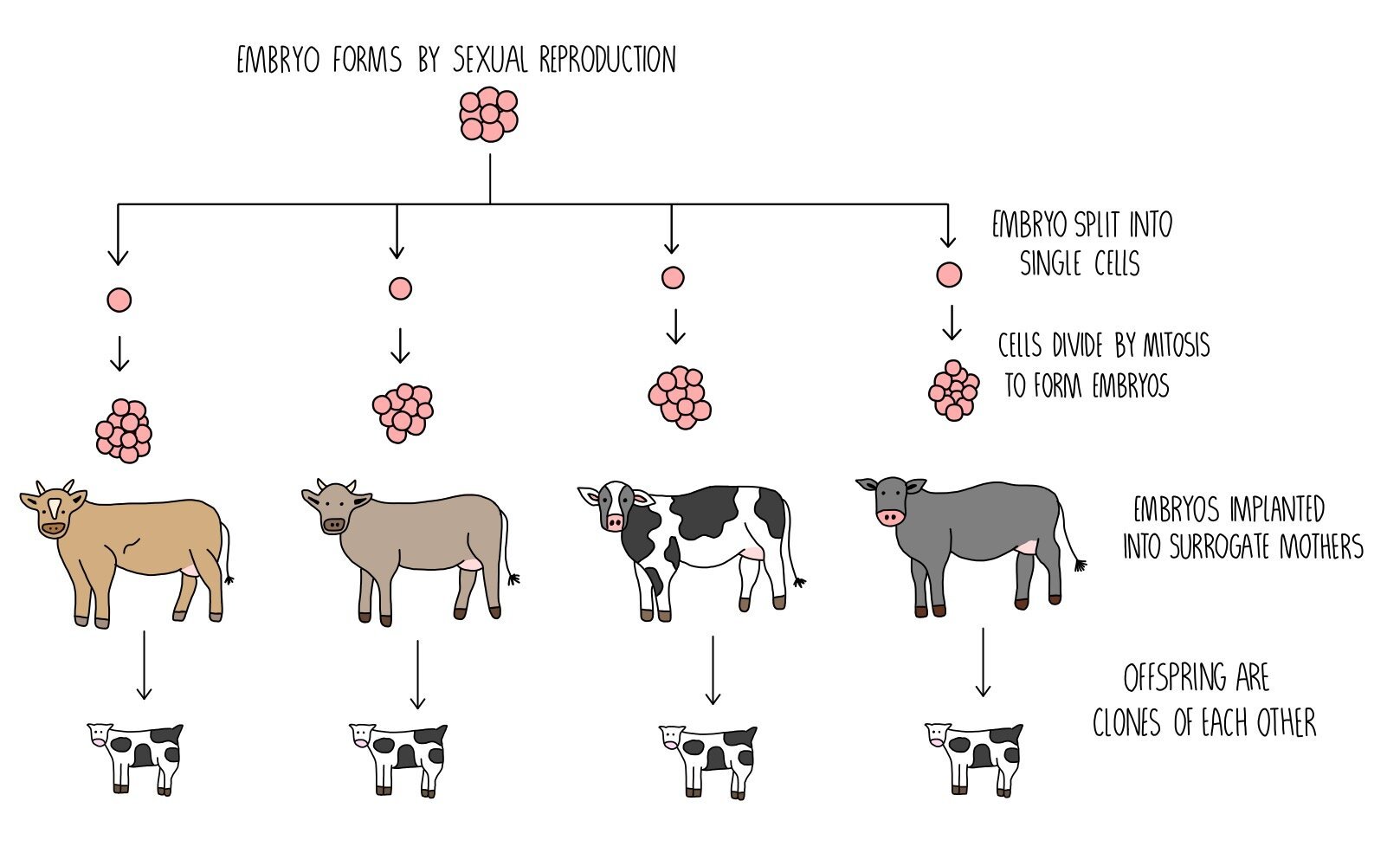
In this process, an egg cell is extracted from a female animal and fertilised in vitro (i.e. by mixing the egg and sperm in a Petri dish). The zygote divides to form an embryo, from which individual cells are removed and placed into a separate Petri dish. Each cell is left to grow and form an embryo, which can be implanted into surrogate mothers to produce multiple clones. They will be clones of each other, but not genetically identical to either of the parents.
Somatic cell nuclear transfer (SCNT)
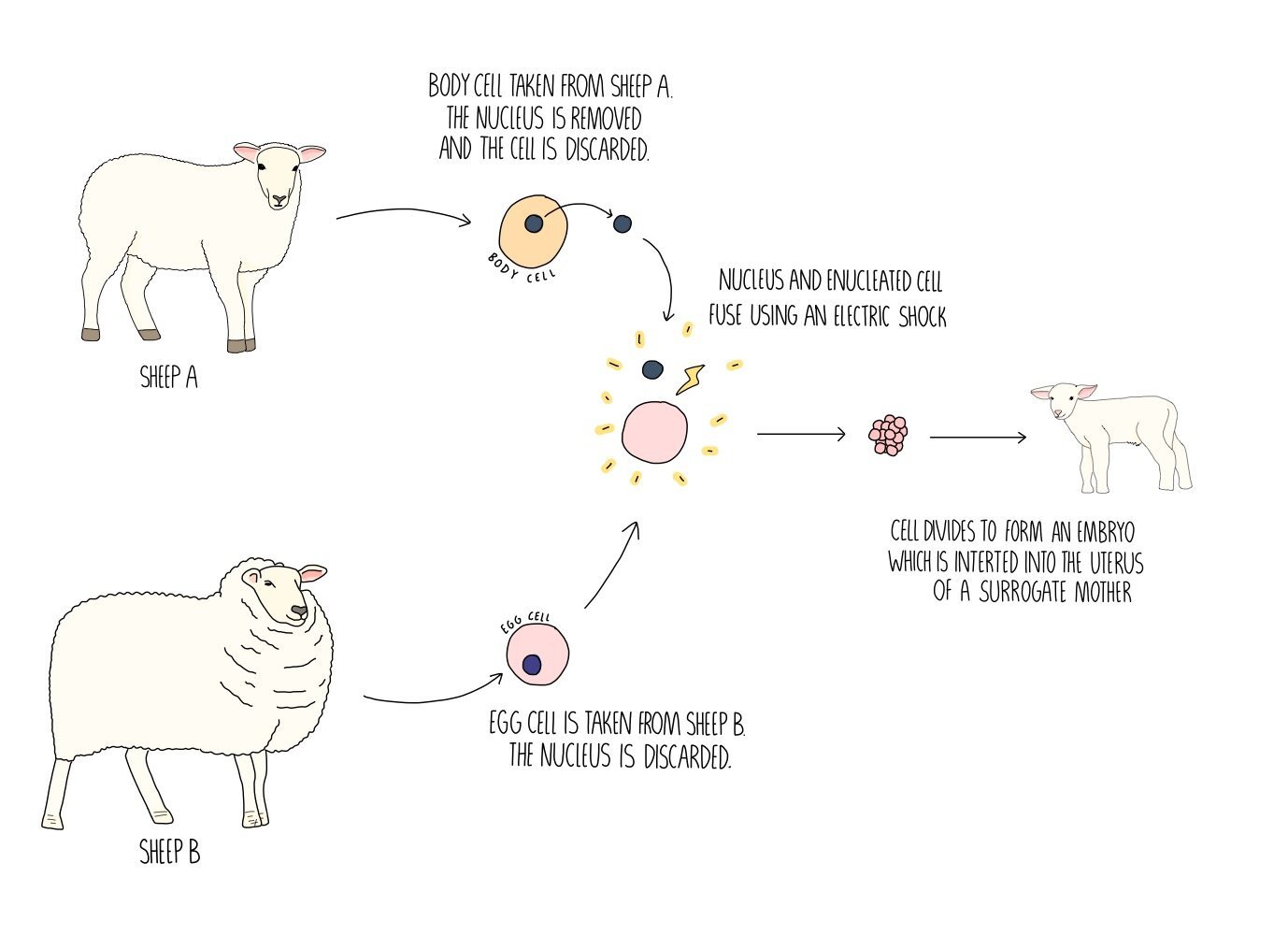
Somatic cell nuclear transfer (SCNT) was used to clone Dolly the sheep in 1996 and has since bene used to create genetically identical organisms for research purposes. It involves taking a somatic cell (any cell that is not a sex cell) and extracting the nucleus. We then take an egg cell from another organism and discard the nucleus to form what’s called an enucleated oocyte. The nucleus from the somatic cell is inserted into the enucleated oocyte. They’re fused together using a technique called electrofusion, which involves applying an electric current. This also stimulates the egg to divide, forming an embryo. The embryo is implanted into a surrogate mother and is a clone of the animal which provided the nucleus.
Adult cell cloning has several applications:
Saving endangered animals from extinction
Genetically modified organisms that produce a useful substance (for example, insulin-synthesising bacteria) can be cloned to form lots of identical organisms that all produce the same substance.
Farmers can clone animals with desirable characteristics – this increases the pool of suitable animals to breed from.
Cloned animals can be used in research — for example, to test drugs. Since all the organisms are genetically identical, any differences between them must come from the conditions being tested (e.g., variations in drug dosage).
Cloned embryonic stem cells can be used to produce any cell type. These cells can be grown into whole tissues, or even organs, to replace those damaged by disease. If the cloned cells contain the same genetic information as the patient, this reduces the risk of rejection.
Animal cloning has the following advantages:
Can be used to reproduce infertile animals
Preserves biodiversity to increasing the population of endangered species
Can take plant independently of breeding seasons
Can lead to the development of new drugs to treat human diseases.
Any desirable traits in the parent will be present in the clone – this is particularly useful in agriculture.
..and the following disadvantages:
Cloned animals can have developmental problems
Reduces the gene pool - this means there isn’t much genetic differences between organisms, making them equally vulnerable to a certain disease
It is expensive, labour-intensive, and time-consuming
Religious/ethical considerations - do humans have the right to create life?
The use of cloned human embryos as a source of stem cells is controversial — some believe that by destroying an embryo, you are destroying a human life.
Biotechnology
Biotechnology is the use of living organisms—or parts of organisms, such as enzymes—in industrial processes. They can be used to produce drugs and food. It usually involves microorganisms, because they grow rapidly under the right conditions, they’re cheap to grow and can be cultured at any time of the year. Microorganisms are used in the following industrial processes:
Brewing – yeast produces ethanol and carbon dioxide from glucose through anaerobic respiration (fermentation). The glucose comes from whatever grain is added e.g. barley.
Bread – anaerobic respiration of yeast is also used in bread making. The carbon dioxide produced is what makes bread rise.
Cheese – an enzyme called chymosin causes milk to clot, eventually forming cheese. Nowadays, the enzyme is extracted from genetically modified yeast cells. Bacteria such as Lactobacillus and Streptococcus are also used in cheese-making. They convert lactose into lactic acid, which helps cheese to solidify.
Yoghurt – Lactobacillus and Streptococcus are also used in the production of yoghurt. The conversion of lactose to lactic acid causes the milk to thicken into yoghurt.
Insulin – bacteria that have been genetically modified to contain the insulin gene synthesise the hormone. The bacteria are grown in large fermenters until the insulin protein is purified. The drug is given to people with type 1 diabetes.
Penicillin – penicillin is an antibiotic that is naturally produced by Penicillium fungi under stress. The fungus is grown in huge industrial fermenters under stress conditions, then the antibiotic is purified and used to treat bacterial infections in people.
The advantages of using microorganisms for food production:
Microorganisms can be grown quickly and cheaply. Since they require simple growth conditions (warm temperatures, glucose, suitable pH), production costs are low.
Compared to rearing livestock for food, microorganisms require less space.
Protein produced by microbes e.g. Quorn is often healthier than meat.
Can be grown in any climate and at any time of the year.
Microorganisms are often grown on waste material, such as molasses. It’s a good way of getting rid of unwanted waste products.
The disadvantages of using microorganisms for food production:
Protein produced by microbes doesn’t have the same taste or texture as meat products.
People may not like the idea of eating something grown on waste products.
Contamination with other microorganisms can spoil the food or lead to health risks.
Consumption of large quantities of protein produced from microorganisms can lead to high uric acid levels, due to the breakdown of excess amino acids.
Industrial fermenters
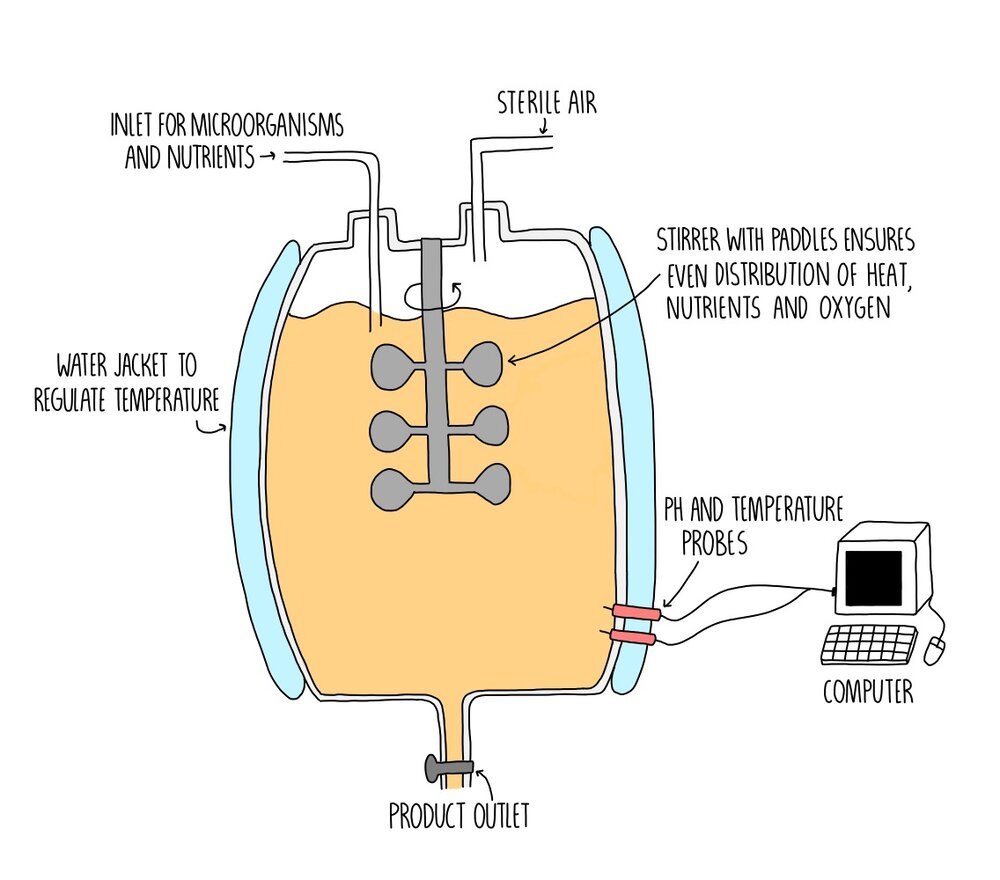
Bacteria and fungi, such as Lactobacillus and yeast, can be grown on a large scale inside industrial fermenters. The fermenters are made from stainless steel (which does not corrode) and are sterilised using hot steam to remove any contaminating microorganisms which may kill or compete the bacteria or fungi. The air inlet provides a supply of oxygen to allow the microorganisms to respire aerobically and paddles to distribute the nutrients and oxygen evenly. The temperature can be carefully controlled using a thermostat and a water jacket. pH is also kept constant using a pH sensor which ensures that the pH is at an optimum value for enzymes within bacteria and fungi to function efficiently.
There are two main methods of culturing microorganisms in fermenters:
Batch fermentation – microorganisms are grown in individual batches. When one batch has finished growing, the vessel is emptied, cleaned then the next batch of microorganisms is introduced. This is known as a closed culture.
Continuous fermentation – microorganisms are grown continually. Nutrients are added and waste products removed while the organisms grow. This is an open culture.
Standard growth curve
Microorganisms growing in a closed culture follow a standard growth curve:
Lag phase – slow growth as the microorganisms need to synthesise the enzymes and other molecules needed to reproduce.
Exponential phase – growth rate increases because conditions are favourable to reproduction. There is plenty of nutrients and a lack of competition for resources. Population size doubles at regular intervals. During this stage, you can calculate how many organisms will be present after a certain number of divisions using the formula: N (population size) = N0 (initial number of organisms) x 2n (where n = number of divisions).
Stationary phase – population size plateaus because the reproductive rate is equal to the death rate. Microorganisms are dying due to reduced nutrient availability and an accumulation of waste products.
Death/decline phase – population size falls because death rate exceeds reproductive rate. Nutrient availability is very low and waste products have reached toxic levels.
Culturing microorganisms
Microorganisms are grown in the lab on agar plates (a sterile Petri dish filled with agar jelly). A wire inoculation loop is sterilised by placing in a Bunsen flame then used to transfer microorganisms (usually from a flask of broth) to the agar plate. The plates are incubated at 25 degrees to allow the microorganisms to grow. Nutrients can be added to the agar jelly to increase growth rate.
Aseptic techniques
When carrying out experiments involving bacteria, it is important to carry out aseptic (sterile) techniques. This is because bacteria are everywhere - all over your hands, in your breath and covering the lab surface - and you don’t want cross-contamination to interfere with your results.
Aseptic techniques include the following:
Use of sterile equipment - this can be done using a machine called an autoclave which looks like a dishwasher but uses steam and high pressure to sterilise the equipment.
Disinfect the lab bench and work surfaces.
Wear gloves.
Place a Bunsen burner near to your workspace when transferring bacteria from one container to another. The heat from the flame draws any microorganisms in the air away from the workspace because hot air rises.
Close windows and doors to prevent draughts blowing microbes towards you.
Investigating the factors that affect microorganism growth:
You can investigate the effects of different factors (such as temperature) on the growth rate of microorganisms using the following experimental method:
- Use a sterile pipette to transfer a fixed volume of bacterial broth to six agar plates.
- Use a sterile plastic spreader to spread the broth across the entire surface of the agar.
- Put the lid on the agar plates and lightly tape them shut.
- Incubate half of the plates in the fridge (at 4oC) and the other half in a 25oC incubator (or left out at room temperature). Place the plate upside down to prevent condensation from dropping onto the agar.
- Place another agar plate (without bacteria) in the fridge, and another in the warm incubator. These are your negative controls – any bacteria found growing on them indicates possible contamination.
- Incubate the plates for a set time period (e.g. 48 hours).
- You should see bacterial colonies on the six plates (but not the two controls). Count the number of colonies formed on each plate and calculate the mean number of colonies at each temperature. You’d expect to find a higher mean number of colonies on the plates incubated at 25oC.
Immobilised enzymes
When using enzymes to catalyse industrial processes, like the breakdown of lactose in the manufacture of lactose-free milk, it’s often useful to have the enzyme fixed, or immobilised, to a surface. This saves you from having to separate the enzyme from the reaction mixture, which can be tricky and expensive. It works by pouring the substrate into a column of immobilised enzymes. Inside the column, the substrate binds to the immobilised enzyme’s active site, just as it would in a normal enzyme-substrate reaction. The mixture flowing out of the column will only contain the desired product.
Enzymes immobilised in alginate beads. Credit: Tess Watson (Flickr)
Enzymes are immobilised in three main ways:
Entrapment - within a silica-gel matrix
Covalently bonding to collagen or cellulose fibres
Encapsulation – inside alginate beads
The advantages of immobilising enzymes are:
The enzyme can be reused – this keeps costs down.
No separation/purification of the enzyme and product is required – this also reduces costs.
The enzyme is protected by the surface it’s attached to – this makes it less likely to denature.
But there are also a few disadvantages:
Compared to free enzymes, they’re more expensive.
Reaction rates can sometimes be lower since the enzyme cannot move around and freely mix with the substrate.
Extra equipment may be needed, which increases costs.
Immobilised enzymes are used in some important industrial processes:
An enzyme called glucose isomerase is used to make high-fructose corn syrup. It converts glucose into another six-carbon sugar called fructose. Fructose is sweeter than glucose, so it makes foods taste sweeter without having to add more sugar. It is commonly found in processed foods.
The enzyme lactase is used in the production of lactose-free milk. It breaks down lactose into its two monosaccharides, glucose and galactose. The product is then suitable for people with lactose intolerance.
The enzyme aminoacylase is used to convert one type of amino acid into another. Amino acids come in two forms, or isomers, known as L- and D-amino acids. Amino acids found in the body come in the L-form, but synthetic amino acids will consist of a mixture of the two isomers. Immobilised aminoacylase converts D-amino acids into L, to be sold as dietary