Elements, compounds and mixtures
One type of atom is called an element. If it forms bonds with other elements, it’s now a compound. Stir that up with something else and you’ve got yourself a mixture.
Elements
- An element is a substance made up of only one type of atom.
- Elements are listed on the Periodic table — they include oxygen molecules (O2), helium atoms (He) and hydrogen molecules (H2). Notice that all of these things are only made up of a single type of atom.
Compounds
- Compounds are made up of more than one type of atom chemically bonded together.
- Examples include carbon dioxide (CO2), water (H2O) and ethanol (C2H5OH).
Mixtures
- A mixture is a group of different elements or compounds which are not chemically bonded together.
- Air is a mixture, as it is made up of nitrogen, oxygen and argon (all elements) along with carbon dioxide and water vapour (compounds).
- Other mixtures include cake batter and shampoo.
- Unlike elements and compounds, which have fixed melting and boiling points, mixtures will melt and boil over a range of different temperatures, as each component of the mixture will change state at different temperatures.
- Mixtures can be separated by filtration, distillation, crystallisation or chromatography. The technique used depends on the nature of substances which make up the mixture.
Filtration
Filtration can be used to separate an insoluble solid from a liquid, such as removing sand from a mixture of sand and water.
To filter something, line a funnel with a sheet of filter paper and pour the mixture into the funnel. The filter paper contains tiny holes, which are small enough to allow the liquid to pass through but will retain any solid particles. The liquid which is collected is called the filtrate.
Simple distillation
Simple distillation is used to separate a liquid from a solution. For example, it could be used to remove water from a mixture of salt and water. The solution is placed in a flask and heated from underneath. When the solvent evaporates, it passes into a condenser which causes the condensation of the solvent gas into a liquid, which can then be collected in a beaker.
Fractional distillation
Fractional distillation is a similar technique to simple distillation, but is used to separate multiple liquids from a mixture. It is used in the separation of different liquid fuels from crude oil, which works because each liquid has a different boiling point.
The crude oil is vaporised and passed into a fractionating column, which is hot at the bottom and cool at the top. The molecules float up the column and condense when the temperature inside the column equals their boiling point. The liquids are removed at different heights along the column and each have slightly different uses.
Crystallisation
Crystallisation is used to form solid crystals from a solution, such as crystals of copper sulfate from copper sulfate solution. To do this, the solvent is slowly evaporated by gently heating the solution. The remaining solution is left to cool to allow the crystals to form and any remaining solution is filtered off. The crystals are washed and dried (either in a warm oven or air-dried).
Paper chromatography
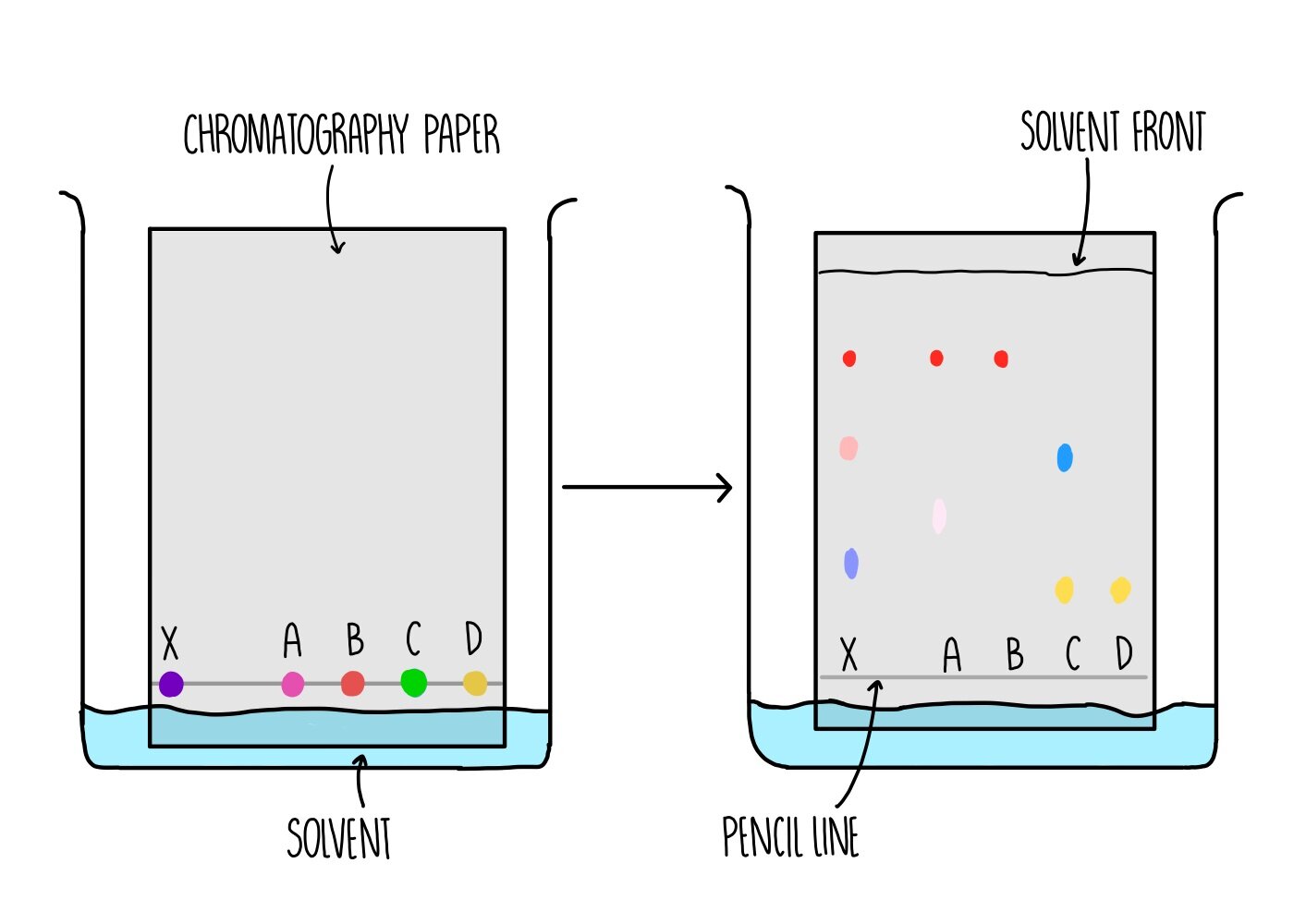
Paper chromatography is used to separate a mixture of soluble substances, such as separating dyes within food colouring or plant pigments. It involves a stationary phase (the part of the equipment which doesn’t move) which is a sheet of paper and a mobile phase (the thing which moves) which is the solvent.
A line is drawn near to the bottom of the piece of paper - this is the starting line where the dyes will be placed. It is important that the line is drawn in pencil because the ink from pens will also dissolve in the solvent and cause a mess.
The paper is placed in a tank containing some solvent and a lid is placed on top of the tank to prevent evaporation of the solvent. The solvent need to be below the starting line so that the dyes do not dissolve in the solvent before they can move along the paper.
As the paper absorbs the solvent, the solvent moves further up the stationary phase. The dyes are carried up the paper along with the solvent with different dyes moving up the paper to different extents. The total distance travelled by the solvent is the solvent front.
An Rf value is calculated for each dye using the equation:
Rf = distance travelled by dye / distance travelled by solvent