Gases in the Atmosphere and Global Warming
The Earth’s atmosphere is a thick layer of air which protects our planet from intense radiation from the Sun. The composition of atmosphere is changing as human activities release carbon dioxide, resulting in the detrimental consequences of global warming.
Composition of the atmosphere
Our atmosphere is mainly composed of four gases in the following proportions:
78% nitrogen
21% oxygen
0.9% argon
0.04% carbon dioxide
Determining the volume of oxygen in air experimentally
The percentage of oxygen in the air can be measured by placing a metal (such as iron) in a measuring cylinder containing a known volume of air. The iron will react with the oxygen to form iron oxide (or rust), reducing the volume of air in the measuring cylinder. The difference in the volume before and after the reaction will be the volume of oxygen that was present in the air. The method for carrying out this experiment is as follows:
Soak a ball of iron wool into acetic acid (which will act as a catalyst) and place in the bottom of a measuring cylinder
Place the measuring cylinder upside-down in a beaker of water
Record the volume of air in the measuring cylinder
As the iron reacts with the oxygen in the air, the water will begin to move up the measuring cylinder.
When the water level stops moving, record the final volume of air in the measuring cylinder
Use the following equation to calculate the percentage of oxygen. You will expect an answer of around 20%
Another method for measuring the percentage of oxygen in air is using gas syringes containing a non-metal such as phosphorous. The method for this type of experiment is described below:
A glass tube containing phosphorous is attached to two gas syringes, one at either end.
One of the gas syringes should be filled with air with the other syringe left empty. Record the total volume of air in the filled syringe.
Heat the phosphorous using a bunsen burner and push the filled syringe to pass air over the phosphorous. The phopshorous will react with the oxygen in the air to form phosphorous oxide.
As oxygen reacts, the volume of air in the syringe will decrease. Once you have completely pushed the air into the other syringe you can record the final volume.
Calculate the percentage of oxygen using the same equation as for the iron wool experiment. Again, you should expect an answer of approximately 20%.
Combustion of elements in oxygen
When we burn something, this is exactly the same thing as reacting it with oxygen. A fire will go out if there is no oxygen to react with it. An element will react with oxygen to form an oxide. Below, we will look at the combustion reactions of three different elements - hydrogen, sulfur and magnesium.
When magnesium reacts with oxygen, it produces a bright white flame. Image: science photo library.
Hydrogen: hydrogen reacts readily with oxygen to form water. This reaction can be explosive which is why hydrogen fuel needs to be carefully stored. It burns with an orange flame and a squeaky pop when burnt in small quantities.
Sulfur: sulfur burns in oxygen with a pale blue flame. This forms sulfur dioxide which is acidic when dissolved in water and is responsible for the formation of acid rain.
Magnesium: magnesium burns with a bright white flame, forming magnesium oxide. Magnesium oxide is basic because it forms an alkali (magnesium hydroxide) when dissolved in water. It is used as a treatment for neutralising stomach acid.
Producing carbon dioxide by thermal decomposition
Thermal decomposition basically means breaking something down using heat. When metal carbonates are heated, they will decompose to form a metal oxide and carbon dioxide. For example, the thermal decomposition of copper carbonate will produce copper oxide and carbon dioxide. You will see a colour change from green to black as copper carbonate decomposes into its oxide. The equation for the reaction is:
Carbon dioxide is a greenhouse gas
The Sun emits heat energy in the form of infrared radiation towards the Earth’s surface. Some of this heat is absorbed but some is reflected back out towards space. Greenhouse gases, such as carbon dioxide can trap some of this reflected infrared radiation, causing the Earth to become gradually warmer. Human activities, such as deforestation and burning fossil fuels has increased the levels of carbon dioxide present in our atmosphere.
A warming planet will cause the polar ice caps to melt, resulting in flooding and destruction of habitats which could have a destructive effect on many species and the rest of the food chain which depend on them. Global warming will also result in extreme weather events such as droughts, cyclones and hurricanes.
Contribution of human activity
Two of the main greenhouse gases are carbon dioxide and methane. Various human activities have contributed to the levels of these gases in the atmosphere, including:
Deforestation – trees absorb carbon dioxide for photosynthesis and lock it in organic molecules like glucose. By removing huge areas of trees, we have reduced the amount of carbon dioxide that is removed from the atmosphere through photosynthesis.
Cattle farming – cattle belch methane gas and excessive levels of cattle farming has contributed to a large increase in methane in the atmosphere. Deforestation and cattle farming are intertwined – huge areas of the Amazon rainforest have been removed to make space for cattle farming.
Burning fossil fuels – we have been extracting and burning fossil fuels such as coal, oil and natural gas. The combustion of these fuels releases carbon dioxide into the atmosphere.
Over the past few decades, scientists have collecting evidence to support the fact that these activities have led to global warming and climate change. This evidence has been reviewed by other scientists in a process called peer review. This is when leading scientists in the field check that the results are valid and that the experiment has been conducted properly. If the evidence passes the peer review stage, it will then be published in a scientific journal. Evidence for climate change can be extrapolated and used to make models and predictions about how temperatures may change in the future. However, because weather patterns are very complex, models on future climate change may be inaccurate. This has led to simplified models and speculations presented in the media which may not present a full picture.
Global climate change
Increases in greenhouse gases in the atmosphere have resulted in increased global warming and a gradual increase in global temperatures. The shift in temperature is a major cause of climate change, which has several potential consequences:
Melting ice caps and flooding – increasing temperatures are speeding up the melting of ice caps. This is leading to rises in sea levels and can result in the loss of habitats.
Extreme weather events, such as typhoons and hurricanes, resulting in habitat destruction
Patterns of rainfall changing, producing floods or draughts. Climate change means that some parts of the world will get more rain while others will get less. This may mean that habitats are no longer suitable for some organisms, causing them to move. Organisms which are unable to move, such as plants, may die out altogether. This will have a knock-on effect on the rest of the food chain.
Habitats changing – some areas may dry out and turn into desert (desertification), whereas others will become colder and wetter, turning swampy. Again, this will affect the organisms which live there.
These effects show how climate change can easily affect whole food chains. Whole human populations may need to migrate to different parts of the world as sea levels rise and cause major flooding. As habitats change or are lost, biodiversity will decrease which will have a negative impact on the development of new medicines and the economy.
Reducing our carbon footprint
The carbon footprint describes the total amount of carbon dioxide and other greenhouse gases emitted during the lifecycle of a product, service or event. For example, the carbon footprint of a aeroplane flight is much larger than that of a train journey, which in turn is larger than a bike ride. Sometimes we talk about the carbon footprint of a single individual. In this case, we would be referring to the amount of electricity they have used, the amount of petrol that have used and the amount of beef they have eaten (since cattle farming is a major contribution to methane emissions).
A person’s carbon footprint can be minimised by reducing the production of carbon dioxide and methane. This may be done by:
Using public transport instead of a car journey
Reducing flights
Reducing the amount of beef in their diet
Turning off electrical devices when not in use
Adding solar panels to their house
Insulating their home to reduce central heating
Pollutants
Much of the pollution in the atmosphere comes from burning fuels such as coal and petrol. These fuels contain hydrocarbons and sulfur impurities. When the fuels are burnt, the following gases are produced:
Carbon dioxide
Water vapour
Carbon monoxide
Sulfur dioxide
Nitrogen dioxides
Carbon (soot)
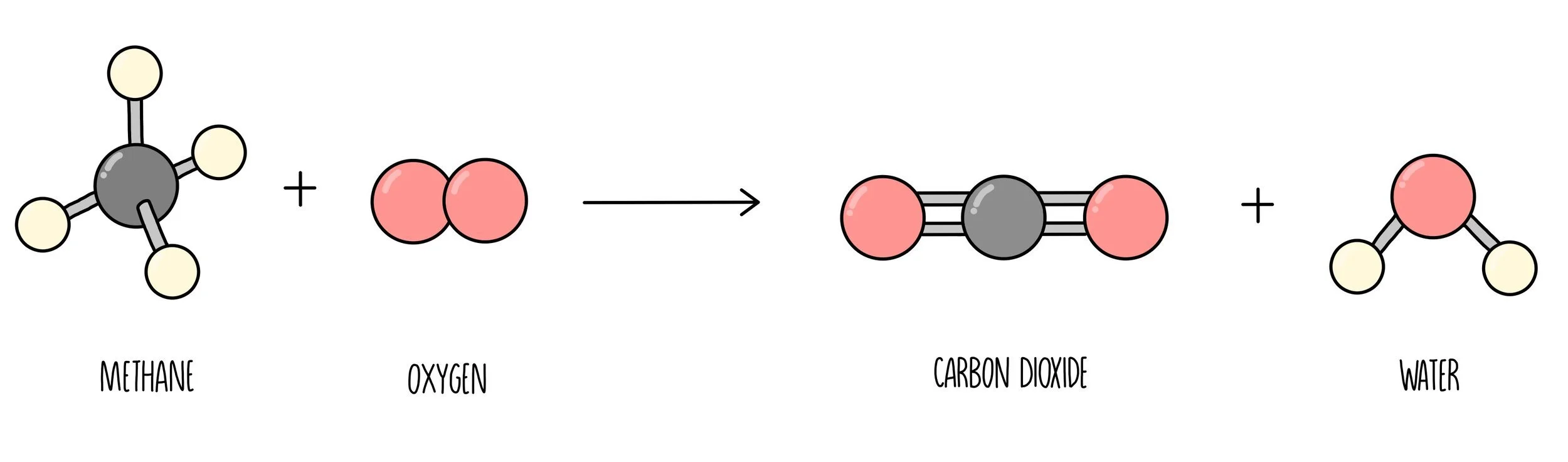
Carbon dioxide and water vapour are formed when the hydrocarbons fully react with oxygen (complete combustion). Both of these products are greenhouse gases.
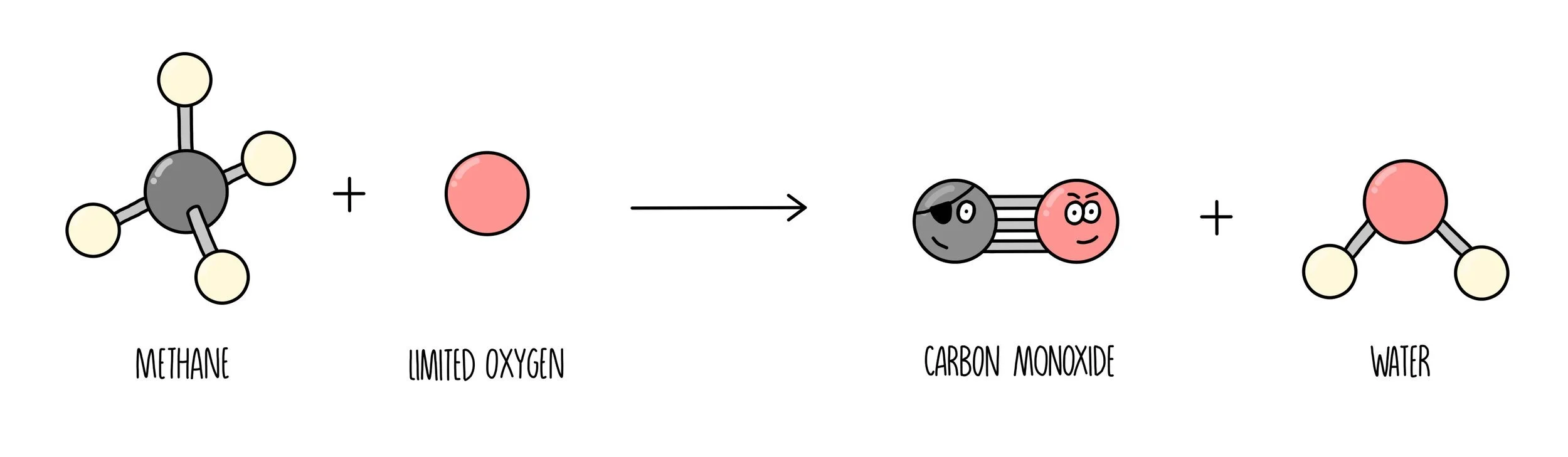
When the supply of oxygen is limited, the hydrocarbons will burn incompletely. Instead of carbon dioxide, carbon monoxide or just carbon (or sometimes both) will be formed instead. Carbon monoxide is poisonous as it binds to haemoglobin inside red blood cells and prevents it from transporting oxygen. It is particularly dangerous because of the fact that it is so hard to detect – it has no colour and no odour so the only way of knowing if there is carbon monoxide is present is by using a carbon monoxide detector.
Carbon (soot) is released as solid black particulates. Particulates can block sunlight from reaching the earth’s surface, in a process called global dimming. Global dimming can have a cooling effect on the earth. Particulates can also cause health problems such asthma.
Sulfur dioxides are formed when the sulfur-containing impurities in coal or petrol react with the oxygen in the air. Sulfur dioxide is bad for the environment because it reacts with water vapour to form acid rain. This causes damage to statues and buildings and lowers the pH of ponds and rivers, harming the organisms living there.
Oxides of nitrogen can also form when fuels burn, due to the reaction between nitrogen and oxygen in the air. Under normal conditions, these two elements don’t react with each other, but when lots of heat is generated during combustion, nitrogen and oxygen have the energy they need to react with each other (activation energy). Just like sulfur dioxide, oxides of nitrogen contribute to acid rain, causing damage to buildings and causing harm to pond life.