Genetic Manipulation
Polymerase Chain Reaction (PCR)
PCR is a technique used to amplify fragments of DNA. This is important because the DNA sample collected at a crime scene doesn’t contain enough material for accurate analysis. Before PCR is carried out, you need to prepare a mixture containing the following:
DNA sample
Free DNA nucleotides
Primers (these are short pieces of DNA which bind to the beginning of the DNA fragment and initiate replication)
The enzyme DNA polymerase which has been extracted from thermophilic (heat-loving) bacteria.
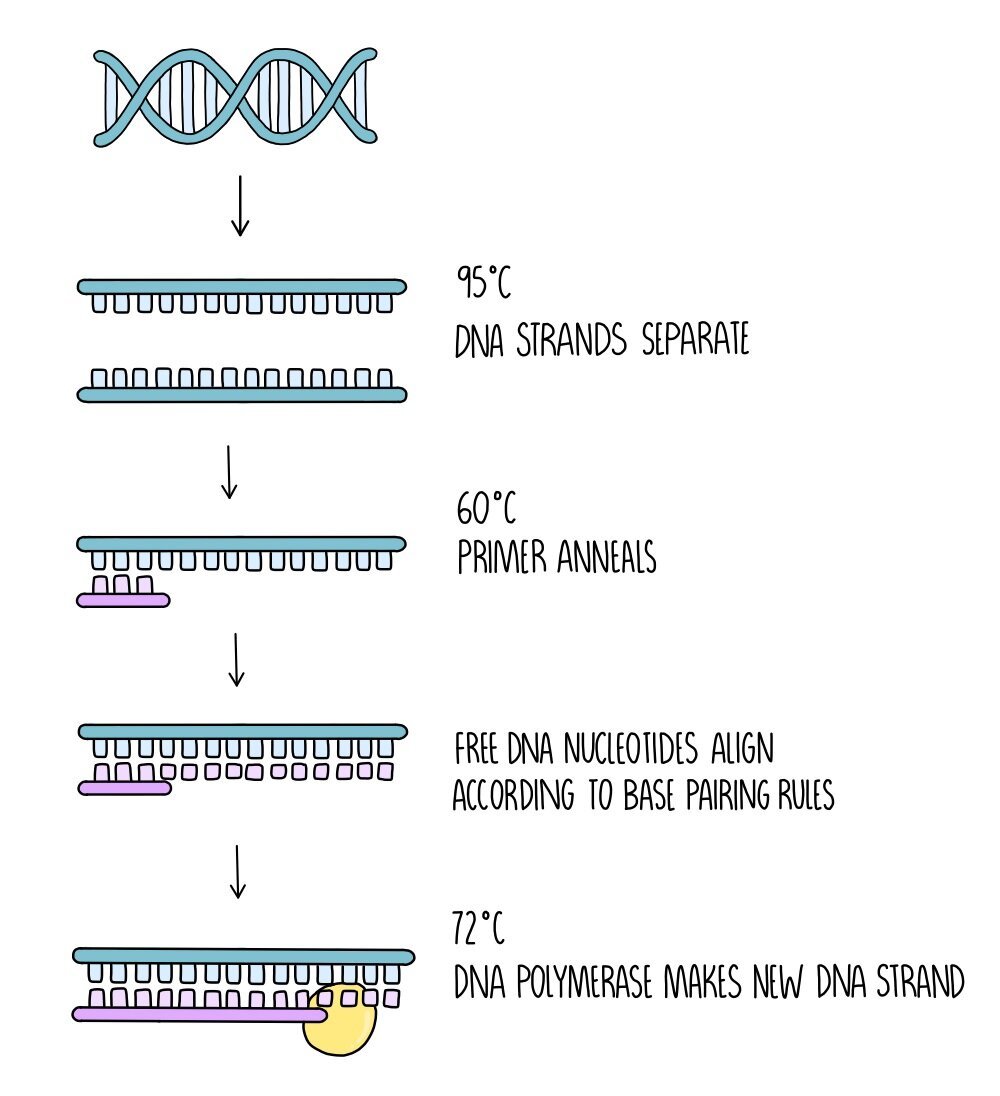
PCR is then carried out in the following stages:
- Separation of the DNA strands - the mixture is heated to 95oC which causes hydrogen bonds between the DNA strands to break.
- Annealing of the primer - the mixture is then cooled to around 60oC which allows the primer to anneal to the DNA.
- DNA synthesis - The temperature is increased to 72oC which is the optimum temperature for DNA polymerase. DNA polymerase forms a new DNA strand from catalysing the formation of phosphodiester bonds between the free DNA nucleotides which align along the DNA template strand by complementary base pairing rules.
Around 30-40 cycles of PCR are carried out, which generates millions of DNA fragments. Each PCR cycle doubles the amount of DNA, so huge numbers of DNA fragments can be quickly generated.
Electrophoresis
So that the DNA can be visualised, we add a fluorescent molecule which binds to the DNA and makes it visible when exposed to UV light. A common fluorescent tag is ethidium bromide, which inserts itself between the DNA bases and gives off fluorescence under UV light.
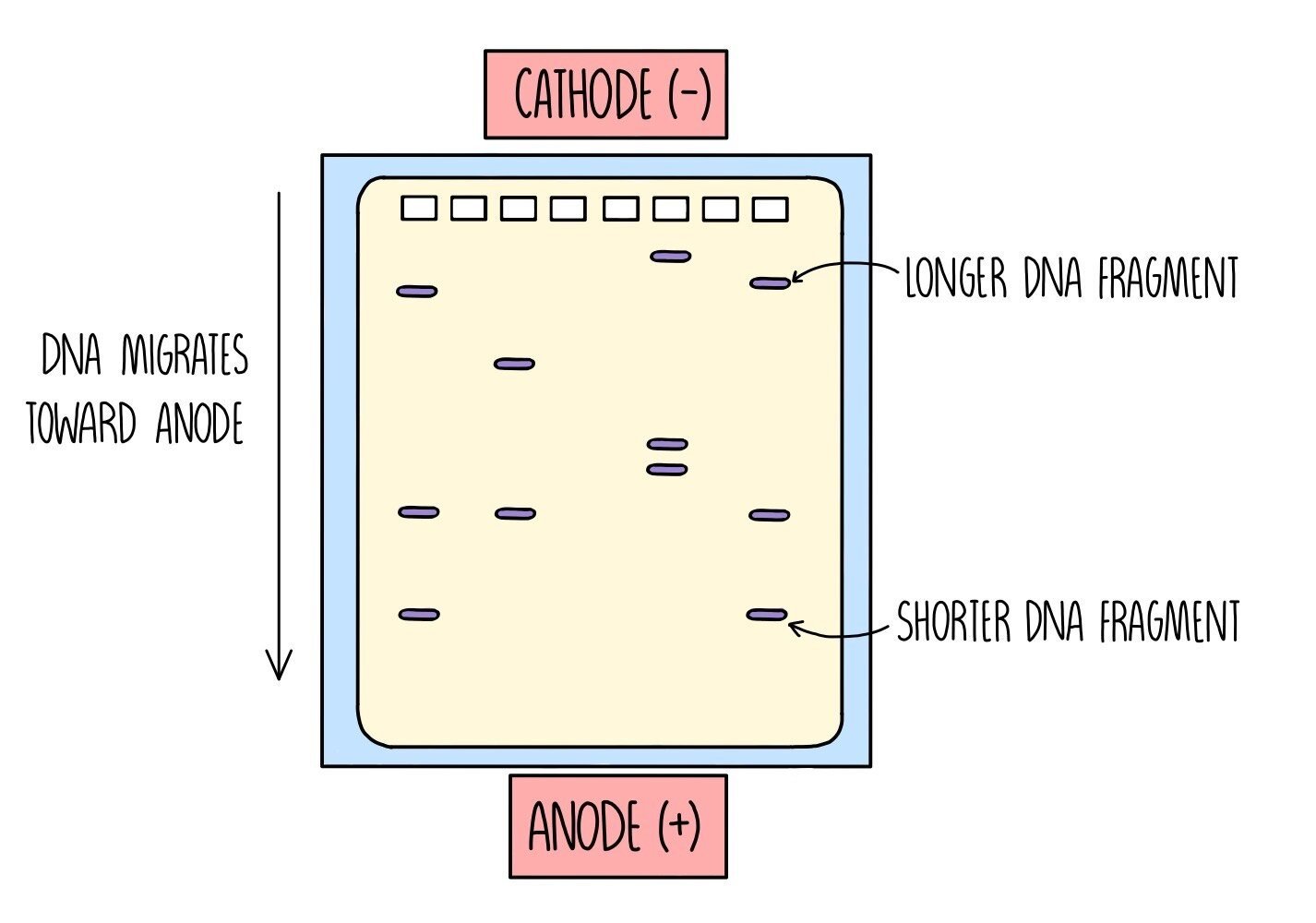
Once the DNA fragments have been amplified and stained with a fluorescent dye, they need to be separated. This is done using gel electrophoresis, which separates the DNA strands according to length. It works because DNA is negatively charged, which means it will move towards a positive charge when placed in an electric field. Shorter DNA fragments travel through the gel more quickly, which means they will travel a longer distance than the larger DNA fragments.
Gel electrophoresis is carried out in the following steps:
An agarose gel is prepared which contains a row of wells at the top of the gel. The gel is placed into a tank containing buffer solution which is able to conduct electricity.
The DNA sample is mixed with a loading dye - this turns the DNA mixture a dark colour and helps you see what you’re doing. A fixed volume of the DNA samples are pipetted into the wells.
An electrical current is passed through the gel and the DNA will begin to move towards the bottom of the gel (towards the anode).
Once the dye has reached the bottom, the electricity is turned off and the banding pattern is visualised under UV light.
Making DNA fragments using restriction enzymes
Restriction enzymes are enzymes that cut double-stranded DNA at specific recognition sequences.
The recognition sites are palindromic – they read the same backwards and forwards.
When the restriction enzyme detects its recognition sequence, it cuts DNA in one of two ways – either straight down the middle, creating ‘blunt ends’ or in a zig-zag fashion, creating ‘sticky ends’. Sticky ends are overhangs of single-stranded DNA at the ends of the fragment. Different restriction enzymes have different recognition sequences. They cut the double-stranded DNA by hydrolysing the phosphodiester backbone.
Scientists will first analyse the DNA to determine which recognition sites are present either side of the gene of interest. They will then use the corresponding enzymes to extract the gene from the longer section of DNA.
DNA profiling
DNA profiling is a technique which can be used to analyse a sample of DNA (e.g. one found at a crime scene) and compare it to DNA samples taken from the suspects.
The DNA sample will be collected (this is usually blood, saliva or semen) and amplified using PCR.
The PCR products are separated using gel electrophoresis, which separates the DNA fragments according to length.
The gel is visualised using UV light and the banding patterns from the suspect’s DNA can be compared with that found at the crime scene.
The same technique can also be used to identify genetic relationships between people (as in paternity testing) or to determine evolutionary relationships between organisms.
Genetic engineering
Drugs such as insulin are made using genetically-engineered bacteria in the following process:
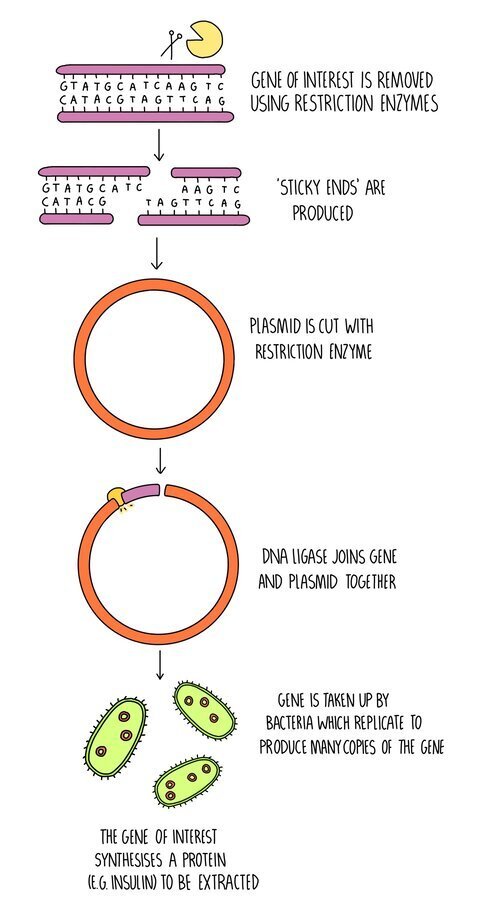
The insulin gene is removed from human DNA using restriction enzymes.
A plasmid is also cut with restriction enzymes.
DNA ligase joins the complementary sticky ends to form the recombinant DNA.
The recombinant plasmid is mixed with bacteria and placed in a machine called an electroporator – this creates an electric field to make the bacterial membrane more permeable.
The transgenic bacteria are grown in large fermenters to produce large amounts of insulin, which can then be extracted.
Bacteria aren’t the only organisms which can be genetically modified to produce drugs - plants and animals can be used too. For genetically modifying plants, a GM bacterium is first made using the process outlined above. The bacterium then acts as a vector, infecting a plant cell and inserting its DNA into the genome of the plant cell. The plant cell grows into an adult plant and all its cells will contain the drug-producing gene. The plant cells will synthesise the protein which can either be extracted or the drug can be delivered to the patient by eating the plant. Cholera vaccines have been made using this method.
Genetically modified animals are produced by injecting the gene for the protein (which will act as the drug) into the nucleus of a fertilised animal egg cell. This is then implanted into an adult animal and as the animal develops, every cell will contain the drug-producing gene. The protein produced from the gene is normally purified from the milk of the animal. This method has been used in goats to produce the drug antithrombin for treating people with defective blood clotting.
Issues with GMOs in medicine and food production
GMOs are not just used for producing drugs, but also for improving agriculture. For example, genes can be added to crop plants to make them resistant to disease or more nutritious. However, the use of GMOs is controversial and there are some arguments for and against their use in medicine and agriculture:
Arguments for the use of GMOs:
Genetic modification can increase crop yield and make food more nutritious - this means that farmers can make more money and people will be healthier.
Genes for pest-resistance can be introduced into crops. This means that farmers save money on pesticides and the environmental problems associated with pesticide use are reduced.
Human proteins produced using GMOs do not result in allergic reactions - for example, insulin as a treatment for diabetes used to be extracted from the blood of pigs and cows. Nowadays human insulin is produced using GM bacteria which doesn’t produce an allergic reaction in patients and is more efficient.
Vaccines produced using genetically-modified plants do not need to be kept cold to stay effective - this is an advantage when vaccines are needed in remote regions where refrigeration is not possible.
Enzymes can be produced using GMOs and are often used in industrial processes e.g. in the manufacture of washing detergents and the textile industry. The use of GMOs makes these processes cheaper.
The process is cheap because once one genetically modified organism is made, lots more can be made through breeding the original GM organism. This makes drugs cheaper.
Arguments against the use of GMOs:
GMOs are often patented, and the seeds are expensive to buy - this puts farmers in developing countries at a disadvantage.
If a GM plant cross-pollinated with another species, it could introduce the genes into other plants with unintended consequences. For example, cross-breeding a herbicide-resistant crop plant with wild plant species could create ‘superweeds’ which are resistant to herbicides. These could out-compete other plants with potential effects on the whole food chain.
There are ethical issues against the use of GMOs - should humans manipulate animals for our own benefit?
Religious reasons - do humans have the right to ‘play God’ and create new life?
Some people worry out the long-term impact of using GMO - there may be unforeseen consequences which are impossible to predict.
Gene therapy
Gene therapy is a method used to treat genetic diseases. For recessive diseases, a functional copy of the gene is introduced into the patient’s cells using a vector (usually viruses or liposomes). Since the cells now contain a working copy of the gene, they will synthesise the functional protein which should reduce the symptoms of the disease. For dominant diseases, the mutated allele can be silenced by inserting a piece of DNA in the middle of the gene to stop it from being expressed.
There are two types of gene therapy:
Germ line – the allele is inserted into a gamete. This has the advantage that all of the cells will contain the functional allele. However, it will be passed onto offspring, making it controversial. It is currently illegal.
Somatic – the allele is inserted into the body cells that are affected by the disorder. It only changes a handful of cells and will need to be repeated when those cells eventually die. It is being used as a treatment in a limited capacity.
Problems with gene therapy:
For somatic gene therapy, the gene would need to be inserted multiple times, as it is expressed in body cells with a limited lifespan. This means that the effects of a single treatment last only a few months or years.
The body may identify the vector carrying the gene as foreign and trigger an immune response.
If the gene becomes inserted in the wrong place in the genome, it may have harmful effects.
Expensive
For germline gene therapy, future generations may inherit the added gene. This poses issues of consent due to its long-term impact.
Ethical issues – some worry that the technology could be used in non-medical areas, e.g., for cosmetic effects.
DNA sequencing: The chain-termination method
The first method developed to sequence DNA was the chain-termination method. It works by amplifying lots of copies of the DNA using PCR, but with varying lengths. The DNA strands are put into size order and the terminal base is read off to determine the overall sequence. Here’s how it’s done:
The DNA sample, primers, DNA polymerase and free nucleotides are mixed in four small tubes (just like with PCR). But unlike standard PCR, modified nucleotides are also added which are attached to a fluorescent tag. In one test tube, fluorescently-labelled adenine is added, while fluorescently-labelled cytosine is added to another, and so on. Once these are incorporated into the newly synthesised DNA chain, no more bases can be added after it.
PCR is carried out, converting the original DNA copy into millions of DNA strands. The strands vary in length due to the addition of fluorescent nucleotides at random positions along the chain.
Electrophoresis separates DNA fragments according to size and are visualised under UV light.
The complementary base sequence can be read off from the gel. The band furthest to the bottom represents the smallest DNA fragment (i.e. one base pair). Reading the gel from the bottom to the top will give you the DNA sequence.
Note that this is the manual version of the chain-termination method. Advances in the field have led to an automatic version of the technique, where all four fluorescent bases are added to the same tube and a machine reads off the sequence, generating a computer readout.
Other developments include high-throughput sequencing and pyrosequencing—where DNA can be sequenced cheaply and thousands (or often, millions) of times faster.
Whole genome sequencing
Sequencing DNA using the chain termination method only works for short strands of DNA. So, to sequence all the DNA in an organism (its genome), scientists fragment it, insert it into bacteria which replicate and amplify the DNA fragments. The DNA is sequenced and put back in order.
Method:
Restriction enzymes cut the genome into fragments
DNA fragments are inserted into man-made plasmids called bacterial artificial chromosomes (BACs)
The BACs are inserted into bacteria so that each bacterium contains a BAC with a different DNA fragment
The bacteria divide to form a colony of identical cells. Each colony will contain the same BAC because they originate from the same bacterium. All the colonies together form what is known as a genomic DNA library.
DNA fragments are purified from each colony and further fragmented using restriction enzymes. This produces overlapping DNA fragments.
The shorter DNA fragments are sequenced using the chain-termination method. Computer software uses the overlapping sequences to put the fragments back in order, generating a complete genomic sequence.
Synthetic biology
If you know the sequence of DNA nucleotides within a gene, you can work out its amino acid sequence and predict the protein’s primary structure. Scientists have used DNA sequences to artificially build proteins from scratch. For example, the antimalarial drug artemisinin is naturally occurring protein in plants. But scientists have been making a synthetic version of the gene, copying the sequenced plant DNA. The synthetic gene is inserted into yeast, which build the protein inside their cells.
Comparing DNA sequences
DNA sequences (either for an individual gene or the entire genome) can be compared between organisms. It has the following applications:
Epidemiology – comparisons between healthy people and those with a disease can help identify genes linked to a certain condition. For example, an allele called APOE4 is linked to an increased risk of developing Alzheimer’s disease.
Evolutionary relationships – comparing DNA sequences of different organisms can provide an insight of how closely related they are in terms of evolutions. For instance, the genomes of humans and chimpanzees are 99% identical, suggesting that we share a recent common ancestor. In contrast, we share roughly 90% of our DNA with dogs, indicating a more distant common ancestor.
Genotype-phenotype relationships – information about an organism’s genotype can allow us to predict its phenotype. Genome sequencing can allow scientists to uncover whether someone will develop Huntington’s disease, for instance, or an increased likelihood of developing breast cancer.