Lattice Enthalpy
Lattice enthalpy
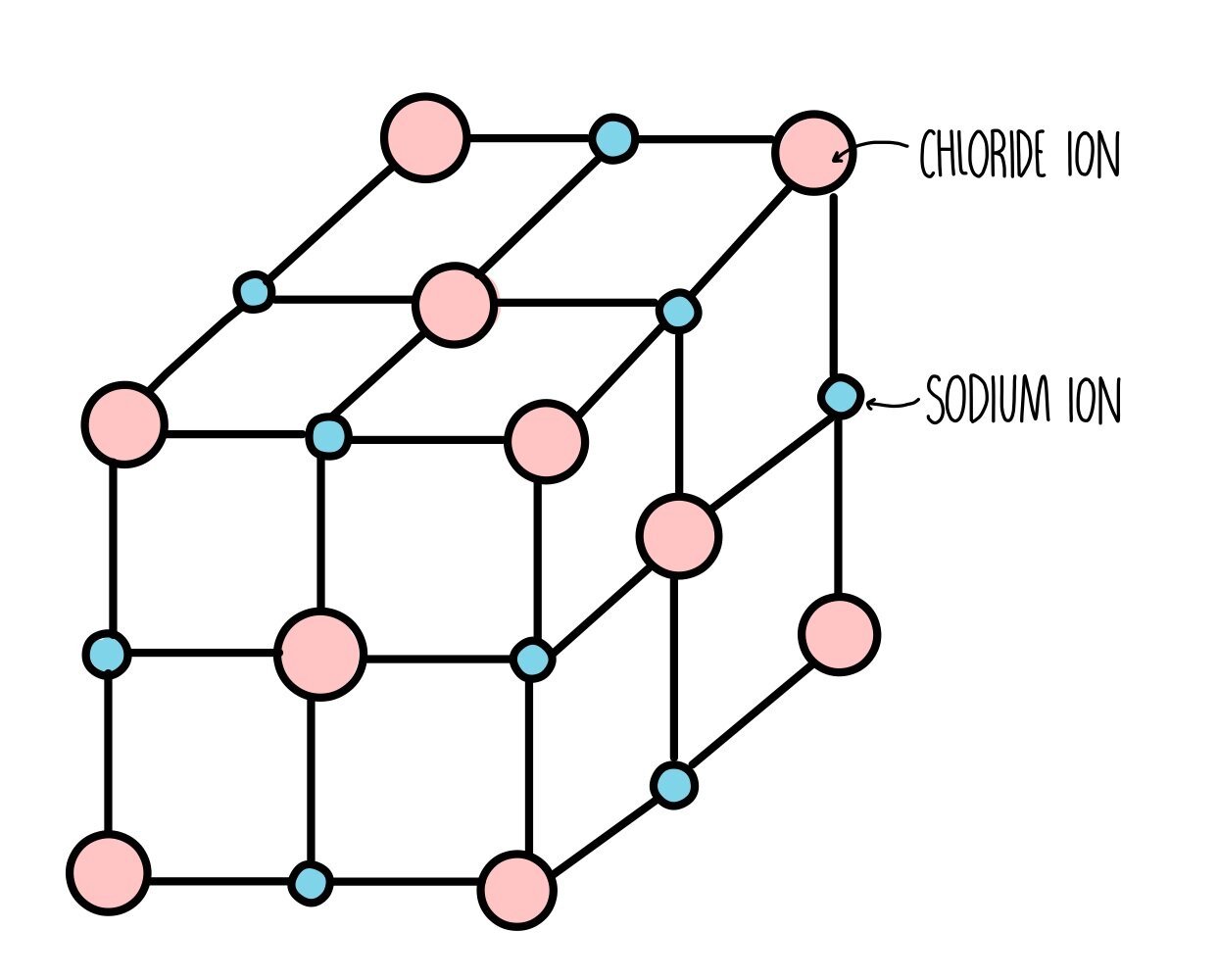
Ionic compounds form huge, regular structures called giant lattice. The ions in the lattice are held together by ionic bonds.
Lattice enthalpy is the energy change associated with the formation of one mole of an ionic lattice from its gaseous ions under standard conditions (298 K and 100 kPa).
The stronger the ionic bonds in the ionic compound, the more negative the lattice enthalpy.
Factors that affect ionic bond strength
The strength of ionic bonds (and therefore lattice enthalpy) depends on two factors:
Ionic charge
Ionic radius
The higher the charge on the ions, the stronger the electrostatic attraction. This means more energy is released when the lattice forms — lattice enthalpy becomes more negative. Therefore, the lattice enthalpy for magnesium chloride is larger (more negative) than sodium chloride, since magnesium ions have a 2+ charge, compared to the 1+ charge on a sodium ion.
The smaller the ionic radius, the closer together the ions are in the lattice and the stronger the ionic bonds that form between them. That means that for smaller ions, there is a more negative lattice enthalpy. Therefore, the lattice enthalpy for lithium fluorine is larger (more negative) than potassium bromide, as the ions that make up the lattice are smaller.
Born-Haber cycles
Lattice enthalpy cannot be directly calculated so we use Born-Haber cycles instead. Bohn-Haber cycles are where the various enthalpy changes are linked together. It relies on Hess’s law – the idea that the total enthalpy change of a reaction is the same regardless of the route taken.
If you look at the Born-Haber cycle for magnesium chloride below, you can see that all of the arrows along the left hand side (enthalpy of formation + atomisation energies + ionisation energies) is equal to the arrows along the right hand side (electron affinity + lattice enthalpy).
Worked example – calculating lattice enthalpy from Born-Haber cycles
There are three things to remember when calculating lattice enthalpy from a Born-Haber cycle:
Ignore the minus sign for enthalpy of formation. If you treat it as a positive value then you can simply add it to the other arrows along the right hand side, to go right from the bottom of the Born-Haber cycle to the top.
Multiply the enthalpy value by the number of particles. In the example below, we have two lots of chlorine atoms. So the atomisation energy and electron affinity for chlorine needs to be multiplied by 2.
Add a negative sign to your final answer. You should end up with a positive value but lattice enthalpies are always negative.
Calculate the lattice enthalpy for magnesium chloride using the Born-Haber cycle.
- Formation of MgCl2 + (atomisation of Cl x 2) + atomisation of Mg + first ionisation energy of Mg + second ionisation energy of Mg = (first electron affinity of Cl x 2) + lattice enthalpy of MgCl2
- 642 + (122 x 2) + 148 + 738 + 1451 = (349 x 2) + LE
- 3223 = 698 + LE
- LE = 3223 – 698 = 2525
- LE = -2525 kJ mol-1
Enthalpy of solution
The enthalpy change of solution is the energy change that takes place when one mole of solute dissolves in water.
Dissolving an ionic lattice in water involves two stages:
The ionic bonds in the lattice break to form gaseous ions. This is an endothermic process.
The gaseous ions form bonds with water molecules and become hydrated. This is an exothermic process and is known as the enthalpy change of hydration.
Substances that release more energy during hydration than the energy required to break the ionic bonds in the first stage are likely to be soluble. For soluble compounds, the enthalpy change of solution will be negative (exothermic).
Calculating enthalpy change of solution
You can calculate the enthalpy change of solution using a Hess cycle, as shown in the worked example below.
Worked example – calculating enthalpy change of solution using a Hess cycle
Calculate the enthalpy change of solution of magnesium chloride, given the following data.
- Lattice enthalpy of MgCl2 = -2526 kJ mol-1
- Enthalpy of hydration of Mg2+ = -1920 kJ mol-1
- Enthalpy of hydration of Cl- = -364 kJ mol-1
Factors which affect the enthalpy change of hydration
We’ve already seen that the second stage of dissolving a compound involves the gaseous ions becoming hydrated i.e. they form bonds with water molecules. The energy released when this happens is known as the enthalpy change of hydration as is defined as:
Enthalpy change of hydration - the energy change that takes place when one mole of gaseous ions dissolves in water.
The enthalpy change of hydration depends on two factors:
The charge on the ion – the larger the charge, the better the ion is at attracting water molecules, forming a stronger electrostatic attraction between them. More energy is released when these stronger bonds are formed, making the enthalpy change of hydration more exothermic (more negative). That means magnesium ions would have a more exothermic enthalpy of hydration compared to potassium ions.
Ionic radius – smaller ions have their charge concentrated in a smaller area (they have a higher charge density). Smaller ions can therefore attract water molecules more easily than larger ones, resulting in a more exothermic enthalpy change of hydration.