Respiration
Aerobic Respiration
Aerobic respiration is made of four stages: glycolysis, the link reaction, the Krebs cycle and oxidative phosphorylation. During aerobic respiration, glucose is effectively burned inside our bodies (it reacts with oxygen) to produce carbon dioxide, water and lots of energy in the form of ATP. The overall equation for aerobic respiration is:
Glycolysis
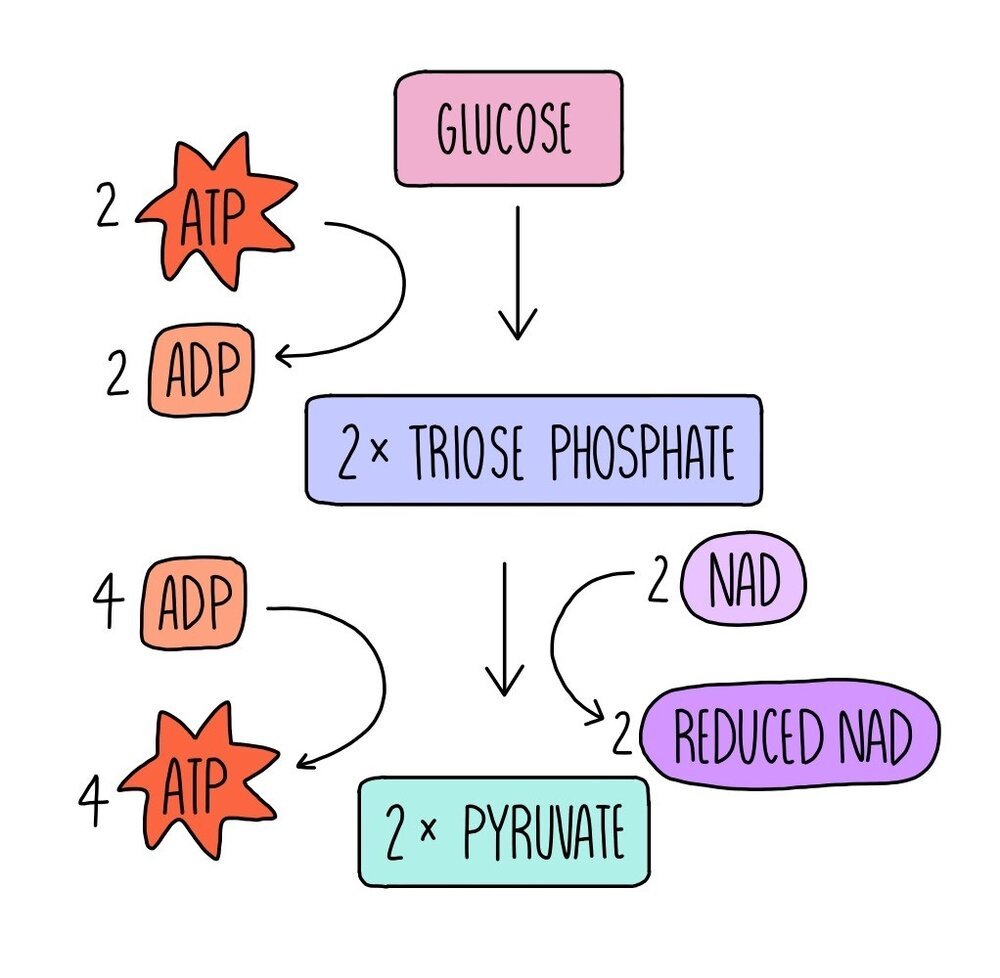
The first stage of aerobic respiration is glycolysis, which takes place in the cytoplasm. Glycolysis converts glucose, a six-carbon molecule, into two smaller three-carbon molecules called pyruvate. This stage doesn’t require oxygen, so it is an anaerobic process and is involved in both aerobic and anaerobic respiration pathways.
Glucose is phosphorylated using the phosphate groups from two molecules of ATP. ATP is hydrolysed into ADP and inorganic phosphate. This forms a molecule which is unstable and immediately breaks down into two three-carbon molecules called triose phosphate (TP). Hydrogen is removed from TP to convert it into pyruvate. The hydrogen is transferred to a coenzyme called NAD to form reduced NAD (NADH). The removal of hydrogen from TP oxidises it. The reduced NAD is used in the last stage of aerobic respiration, oxidative phosphorylation, whereas the pyruvate moves into the mitochondria for the next stage of respiration, the link reaction.
The conversion of triose phosphate to pyruvate produced four molecules of ATP. Since two molecules were used for the phosphorylation of glucose in the first step, this means there is a net gain of two ATP molecules in glycolysis.
The Link Reaction
The link reaction takes place in the mitochondrial matrix and converts pyruvate into a molecule called acetyl coenzyme A (acetyl CoA). This stage does not produce any energy in the form of ATP but does produce reduced NAD and acetyl CoA. Reduced NAD will be used in oxidative phosphorylation while the acetyl CoA will be used in the next stage of aerobic respiration, the Krebs cycle.
During the link reaction, a carbon atom is removed from pyruvate, forming carbon dioxide. This converts pyruvate into a two-carbon molecule called acetate. Hydrogen is also removed from pyruvate in the conversion into acetate, which is picked up by the coenzyme NAD to form reduced NAD. The acetate is combined with coenzyme A (CoA) to form acetyl CoA.
Since one glucose molecule is converted into 2x pyruvate, the link reaction happens twice for every glucose molecule. This means that each molecule of glucose produces two molecules of acetyl CoA (along with 2x carbon dioxide and 2x NADH).
The Krebs cycle
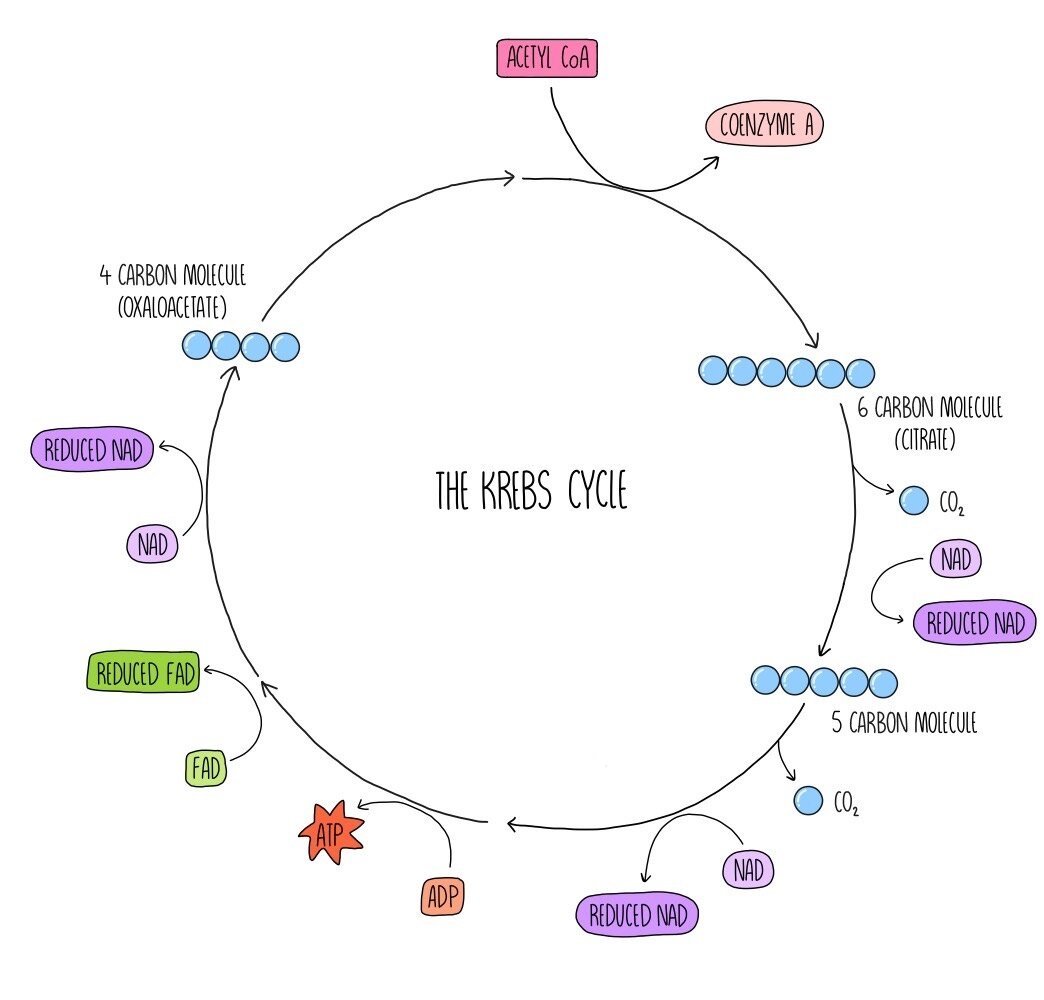
The Krebs cycle (also known as the citric acid cycle) is a series of reactions which generate reduced NAD and a similar molecule called reduced FAD which are needed for oxidative phosphorylation. Acetyl CoA from the link reaction reacts with a four-carbon molecule called oxaloacetate. The coenzyme A portion of acetyl CoA is removed and returns to the link reaction to be reused. A 6-carbon molecule called citrate is produced. Carbon and hydrogen are removed from citrate, forming carbon dioxide and reduced NAD. The citrate is converted into a 5-carbon compound. Decarboxylation and dehydrogenation occur once more, which converts the 5-carbon compounds into the 4-carbon molecule oxaloacetate which we started with. ATP, 2 molecules of reduced NAD, one molecule of FAD and carbon dioxide are also formed in this step. This cycle takes place twice for each glucose molecule that is respired aerobically.
Oxidative Phosphorylation
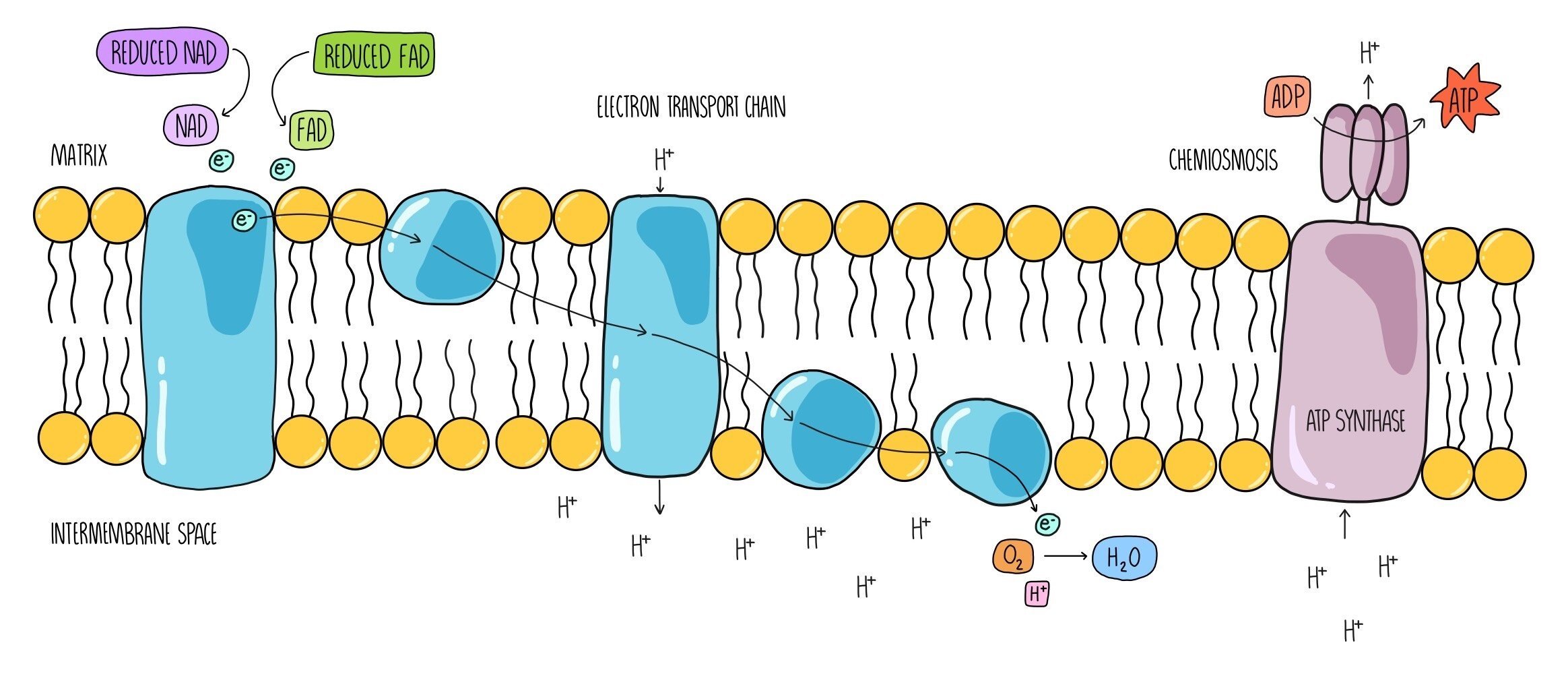
Oxidative phosphorylation is the last stage of aerobic respiration, and it is the part where most of the ATP is made. It uses the electrons that are being carried by reduced NAD and reduced FAD that have been generated in the first three stages. It takes place across the inner mitochondrial membrane and involves two processes - the electron transport chain and chemiosmosis.
The coenzymes reduced NAD and reduced FAD release hydrogen atoms which split into hydrogen ions and electrons. The electrons are passed onto electron carriers which are embedded within the inner mitochondrial membrane and travel along a series of electron carriers known as the electron transport chain. As they travel between the electron carriers, they lose energy. This energy is used by the carriers to pump hydrogen ions from the mitochondrial matrix across the inner membrane. Hydrogen ions accumulate in the intermembrane space, and this generates a proton gradient (sometimes referred to as an electrochemical gradient) across the membrane. Hydrogen ions then flow back into the matrix through the enzyme ATP synthase which uses the movement of hydrogen ions (the proton motive force) to add a phosphate group onto ADP to form ATP. The process by which the movement of hydrogen ions produces ATP is called chemiosmosis. Once the electrons reach the end of the electron transport chain, they are passed onto oxygen, which is referred to as the ‘final electron acceptor’. Oxygen combines with electrons and hydrogen ions to form water, one of the products of aerobic respiration.
Total ATP production
Aerobic respiration produces a total of 38 ATP molecules per one molecule of glucose respired. Here’s a breakdown of the ATP production at each of the different stages. Each molecule of reduced NAD produces 3 ATP and each molecule of reduced FAD produces 2 ATP. Remember that the link reaction and Krebs cycle happen twice for each molecule of glucose, because it is converted into 2x pyruvate.
Glycolysis: direct production of 2 ATP
Glycolysis: 2 reduced NAD are converted into 6 ATP (2 x 3) in oxidative phosphorylation
Link reaction: 2 reduced NAD are converted into 6 ATP (2 x 3) in oxidative phosphorylation
Krebs cycle: direct production of 2 ATP
Krebs cycle: 6 reduced NAD are converted into 18 ATP (6 x 3) in oxidative phosphorylation
Krebs cycle: 2 reduced FAD are converted into 4 ATP (2 x 2) in oxidative phosphorylation
Total ATP = 2 + 6 + 6 + 2 + 18 + 4 = 38 ATP
Anaerobic respiration
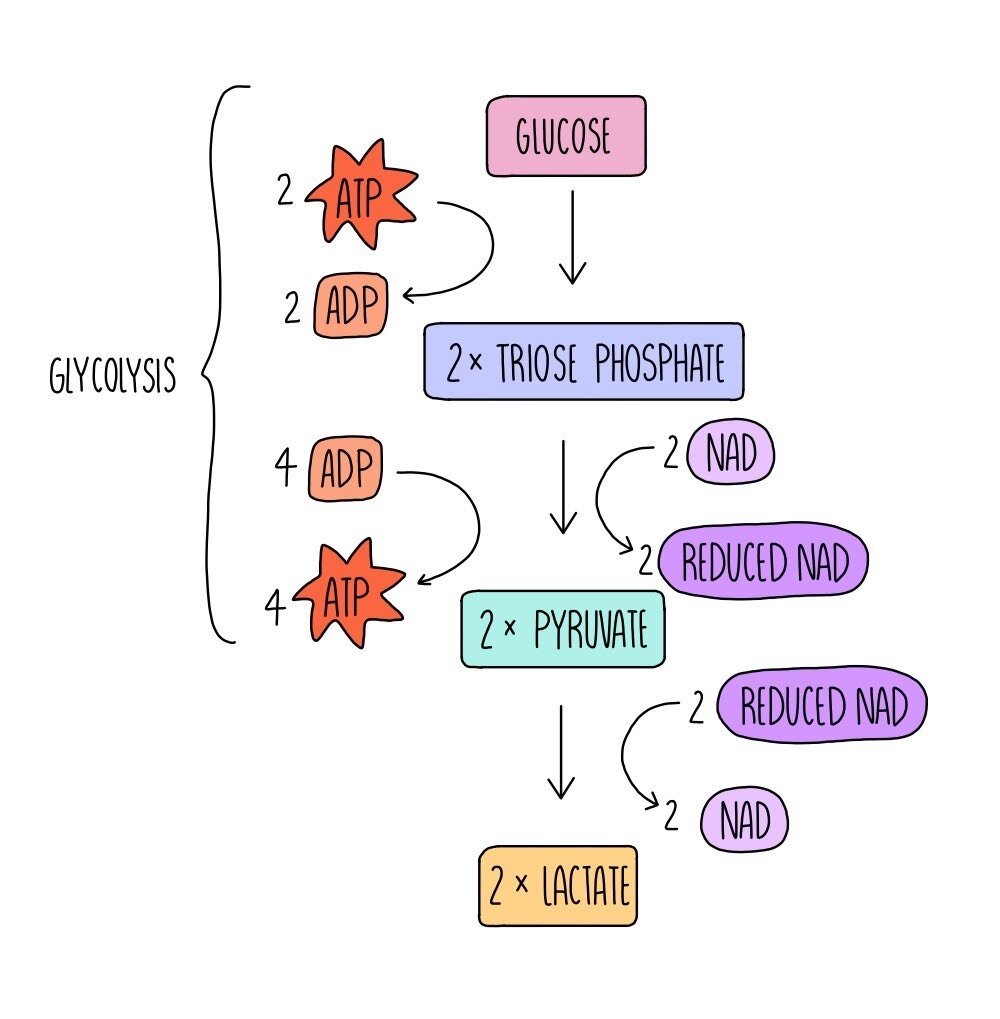
Respiration can also occur in the absence of oxygen - this is called anaerobic respiration. In mammals, glucose can be converted into lactate (aka lactic acid) which releases a small amount of energy in the form of ATP.
The first step of anaerobic respiration is the same as aerobic respiration: glycolysis. Glucose is converted into pyruvate with the net release of 2 ATP molecules. 2 molecules of reduced NAD are also formed. In the second step, reduced NAD donates hydrogen (and electrons) to pyruvate, producing lactate and NAD. This regenerates more oxidised NAD for glycolysis. This enables anaerobic respiration to continue and ensures that small amounts of energy can still be made in the absence of oxygen, allowing biological reactions to keep ticking over.
Continued anaerobic respiration results in the build-up of lactate, which needs to be broken down. Cells can convert lactate back into pyruvate, which is then able to enter aerobic respiration at the Krebs cycle. In addition, liver cells can convert lactate into glucose, which can then be respired aerobically (if oxygen is now present) or stored for later use.
In plants and yeast, anaerobic respiration is a little different. Pyruvate produced in glycolysis is converted into ethanol and carbon dioxide.
Respiratory substrates
As well as respiring glucose, cells also respire other substrates, including carbohydrates, proteins and lipids. Different respiratory substrates release different amounts of energy in respiration: Lipids release the most, followed by proteins and carbohydrates release the least.
Because the majority of ATP is made using the proton gradient that flows through ATP synthase (in oxidative phosphorylation), the more hydrogens a respiratory substrate has, the more energy it can release. Lipids contain the most hydrogens per unit mass while carbohydrates contain the least.
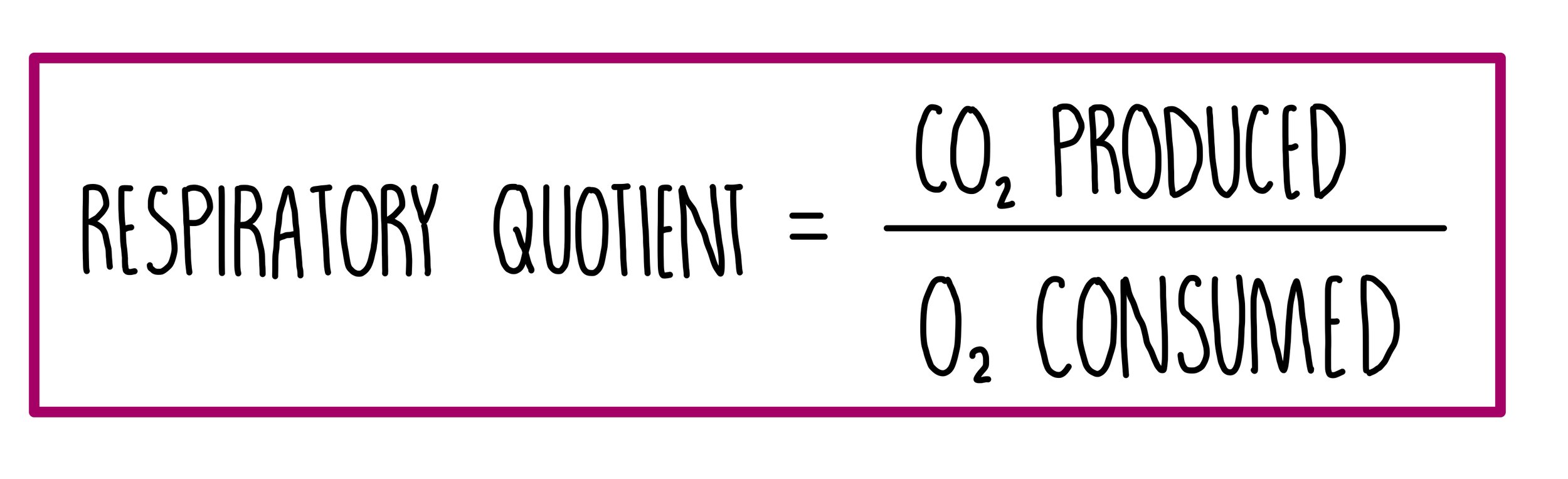
Respiratory quotient
The respiratory quotient (RQ) is the volume of carbon dioxide produced during respiration, divided by the volume of oxygen consumed for a particular respiratory substrate in a given time.
For example, when glucose is used in respiratory, six oxygen molecules are consumed, and six carbon dioxide molecules are produced. This gives a RQ value of 1. Other respiratory substrates, like lipids, have a lower RQ because more oxygen is needed to oxidise fats compared to carbohydrates.
Respiratory quotients tell us what respiratory substrate is being used and whether aerobic or anaerobic respiration is taking place. RQ values greater than 1 indicate a short oxygen supply since the organism is respirating both aerobically and anaerobically.
Measuring the rate of respiration
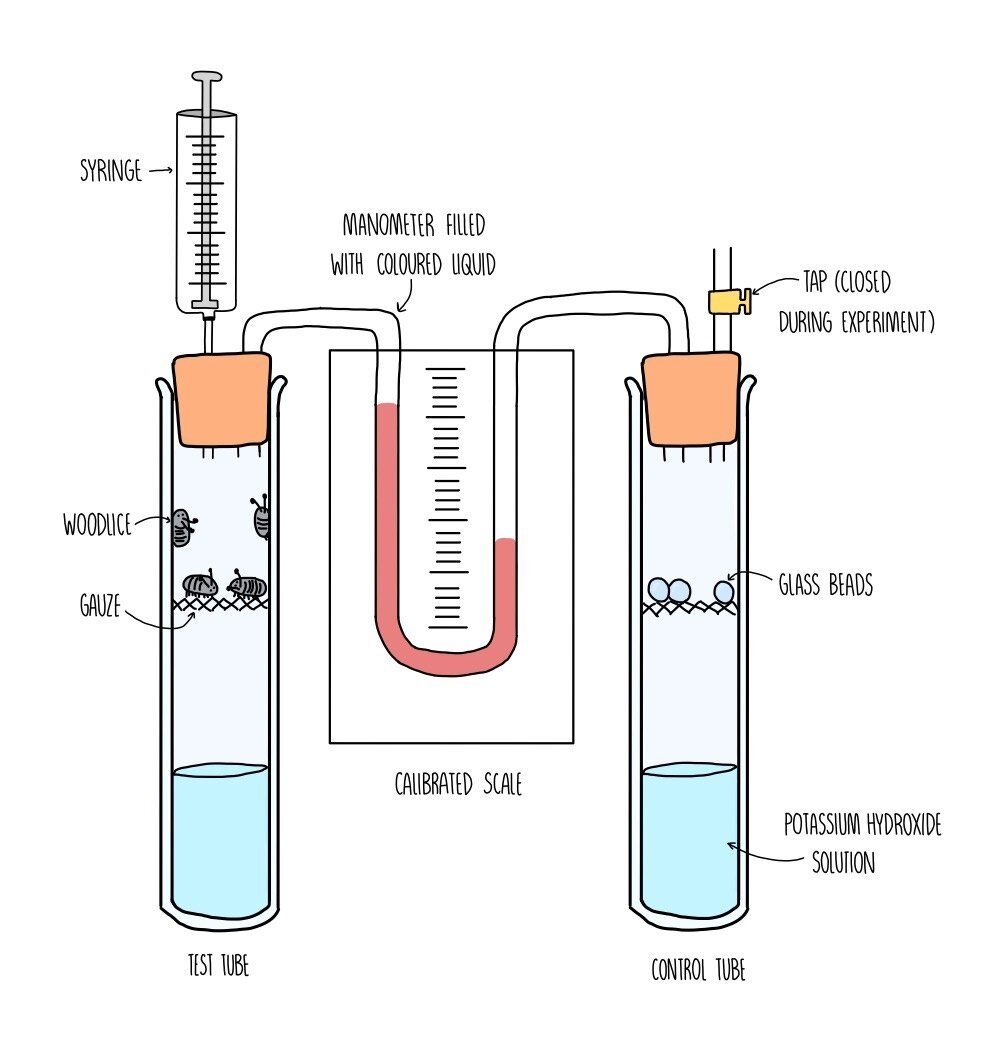
The rate of respiration is measured using a piece of apparatus called a respirometer and works by measuring either the amount of oxygen used up by an organism or the amount of carbon dioxide produced. The faster the amount of oxygen consumed, the faster the rate of respiration.
You would set up the respirometer as shown in the diagram, with respiring organisms (such as woodlice) in one test tube connected to another test tube by a manometer. The manometer contains a coloured liquid which will move closer towards the respiring test tube as oxygen is consumed. The test tube on the right is a control test tube, containing a non-respiring substance, such as glass beads. The purpose of the control tube is to ensure that only respiration is causing the movement of liquid in the manometer. The control tube should be as similar as possible to the test tube e.g., the glass beads should be the same mass as the woodlice. In each test tube you need to add the same volume of potassium hydroxide solution which absorbs carbon dioxide - this ensures that the movement of the liquid is only affected by the decreasing levels of oxygen.
Once the apparatus has been set up, it is left for a certain period of time (e.g., 30 minutes). This will allow for the potassium hydroxide to absorb all the carbon dioxide in the test tubes. You then record the distance moved by the liquid in the manometer in a given time, using the calibrated scale and a stopwatch. You then calculate the volume of oxygen taken in by the woodlice per minute. Repeat the experiment at least three times and calculate a mean.